Post-translational modifications of recombinant human lysyl oxidase-like 2 (rhLOXL2) secreted from Drosophila S2 cells
- PMID: 23319596
- PMCID: PMC3581389
- DOI: 10.1074/jbc.C112.421768
Post-translational modifications of recombinant human lysyl oxidase-like 2 (rhLOXL2) secreted from Drosophila S2 cells
Abstract
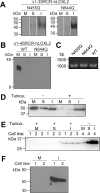
Human lysyl oxidase-like 2 (hLOXL2) is highly up-regulated in metastatic breast cancer cells and tissues and induces epithelial-to-mesenchymal transition, the first step of metastasis/invasion. hloxl2 encodes four N-terminal scavenger receptor cysteine-rich domains and the highly conserved C-terminal lysyl oxidase (LOX) catalytic domain. Here, we assessed the extent of the post-translational modifications of hLOXL2 using truncated recombinant proteins produced in Drosophila S2 cells. The recombinant proteins are soluble, in contrast to LOX, which is consistently reported to require 2-6 m urea for solubilization. The recombinant proteins also show activity in tropoelastin oxidation. After phenylhydrazine derivatization and trypsin digestion, we used mass spectrometry to identify peptides containing the derivatized lysine tyrosylquinone cross-link at Lys-653 and Tyr-689, as well as N-linked glycans at Asn-455 and Asn-644. Disruption of N-glycosylation by site-directed mutagenesis or tunicamycin treatment completely inhibited secretion so that only small quantities of inclusion bodies were detected. The N-glycosylation site at Asn-644 in the LOX catalytic domain is not conserved in human LOX (hLOX), although the LOX catalytic domain of hLOX shares ∼50% identity and ∼70% homology with hLOXL2. The catalytic domain of hLOX was not secreted from S2 cells using the same expression system. These results suggest that the N-glycan at Asn-644 of hLOXL2 enhances the solubility and stability of the LOX catalytic domain.
Figures


Similar articles
-
Crystal structure of human lysyl oxidase-like 2 (hLOXL2) in a precursor state.Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):3828-3833. doi: 10.1073/pnas.1720859115. Epub 2018 Mar 26. Proc Natl Acad Sci U S A. 2018. PMID: 29581294 Free PMC article.
-
Extracellular Processing of Lysyl Oxidase-like 2 and Its Effect on Amine Oxidase Activity.Biochemistry. 2018 Dec 26;57(51):6973-6983. doi: 10.1021/acs.biochem.8b01008. Epub 2018 Dec 13. Biochemistry. 2018. PMID: 30499665 Free PMC article.
-
MCF-7 cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro.J Biol Chem. 2013 Oct 18;288(42):30000-30008. doi: 10.1074/jbc.C113.502310. Epub 2013 Sep 6. J Biol Chem. 2013. PMID: 24014025 Free PMC article.
-
Lysine Deacetylation Is a Key Function of the Lysyl Oxidase Family of Proteins in Cancer.Cancer Res. 2024 Mar 4;84(5):652-658. doi: 10.1158/0008-5472.CAN-23-2625. Cancer Res. 2024. PMID: 38194336 Review.
-
Structural and functional diversity of lysyl oxidase and the LOX-like proteins.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):220-4. doi: 10.1016/s1570-9639(03)00053-0. Biochim Biophys Acta. 2003. PMID: 12686136 Review.
Cited by
-
LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway.Sci Rep. 2017 Mar 23;7:44988. doi: 10.1038/srep44988. Sci Rep. 2017. PMID: 28332555 Free PMC article.
-
Exploring the Interplay between Polyphenols and Lysyl Oxidase Enzymes for Maintaining Extracellular Matrix Homeostasis.Int J Mol Sci. 2023 Jul 1;24(13):10985. doi: 10.3390/ijms241310985. Int J Mol Sci. 2023. PMID: 37446164 Free PMC article. Review.
-
Insight into the Spatial Arrangement of the Lysine Tyrosylquinone and Cu2+ in the Active Site of Lysyl Oxidase-like 2.Int J Mol Sci. 2022 Nov 12;23(22):13966. doi: 10.3390/ijms232213966. Int J Mol Sci. 2022. PMID: 36430446 Free PMC article.
-
Genetic Drivers of Head and Neck Squamous Cell Carcinoma: Aberrant Splicing Events, Mutational Burden, HPV Infection and Future Targets.Genes (Basel). 2021 Mar 15;12(3):422. doi: 10.3390/genes12030422. Genes (Basel). 2021. PMID: 33804181 Free PMC article. Review.
-
Redox Potentials of Disulfide Bonds in LOXL2 Studied by Nonequilibrium Alchemical Simulation.Front Chem. 2021 Dec 14;9:797036. doi: 10.3389/fchem.2021.797036. eCollection 2021. Front Chem. 2021. PMID: 34970534 Free PMC article.
References
-
- Moreno-Bueno G., Salvador F., Martín A., Floristán A., Cuevas E. P., Santos V., Montes A., Morales S., Castilla M. A., Rojo-Sebastián A., Martínez A., Hardisson D., Csiszar K., Portillo F., Peinado H., Palacios J., Cano A. (2011) Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol. Med. 3, 528–544 - PMC - PubMed
-
- Payne S. L., Hendrix M. J., Kirschmann D. A. (2007) Paradoxical roles for lysyl oxidases in cancer–a prospect. J. Cell. Biochem. 101, 1338–1354 - PubMed
-
- Peng L., Ran Y. L., Hu H., Yu L., Liu Q., Zhou Z., Sun Y. M., Sun L. C., Pan J., Sun L. X., Zhao P., Yang Z. H. (2009) Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis 30, 1660–1669 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

