Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction
- PMID: 23319599
- PMCID: PMC3549968
- DOI: 10.1083/jcb.201207036
Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction
Abstract
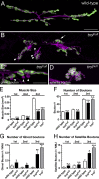
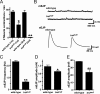
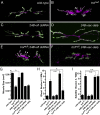
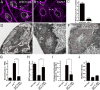
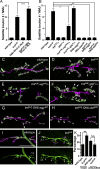
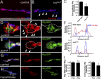
Heparan sulfate proteoglycans (HSPGs) play pivotal roles in the regulation of Wnt signaling activity in several tissues. At the Drosophila melanogaster neuromuscular junction (NMJ), Wnt/Wingless (Wg) regulates the formation of both pre- and postsynaptic structures; however, the mechanism balancing such bidirectional signaling remains elusive. In this paper, we demonstrate that mutations in the gene of a secreted HSPG, perlecan/trol, resulted in diverse postsynaptic defects and overproduction of synaptic boutons at NMJ. The postsynaptic defects, such as reduction in subsynaptic reticulum (SSR), were rescued by the postsynaptic activation of the Frizzled nuclear import Wg pathway. In contrast, overproduction of synaptic boutons was suppressed by the presynaptic down-regulation of the canonical Wg pathway. We also show that Trol was localized in the SSR and promoted postsynaptic accumulation of extracellular Wg proteins. These results suggest that Trol bidirectionally regulates both pre- and postsynaptic activities of Wg by precisely distributing Wg at the NMJ.
Figures



Similar articles
-
Carrier of Wingless (Cow) Regulation of Drosophila Neuromuscular Junction Development.eNeuro. 2020 Mar 10;7(2):ENEURO.0285-19.2020. doi: 10.1523/ENEURO.0285-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32024666 Free PMC article.
-
Heparan sulfate proteoglycans in Drosophila neuromuscular development.Biochim Biophys Acta Gen Subj. 2017 Oct;1861(10):2442-2446. doi: 10.1016/j.bbagen.2017.06.015. Epub 2017 Jun 20. Biochim Biophys Acta Gen Subj. 2017. PMID: 28645846 Review.
-
Notum coordinates synapse development via extracellular regulation of Wingless trans-synaptic signaling.Development. 2017 Oct 1;144(19):3499-3510. doi: 10.1242/dev.148130. Epub 2017 Aug 31. Development. 2017. PMID: 28860114 Free PMC article.
-
Two classes of matrix metalloproteinases reciprocally regulate synaptogenesis.Development. 2016 Jan 1;143(1):75-87. doi: 10.1242/dev.124461. Epub 2015 Nov 24. Development. 2016. PMID: 26603384 Free PMC article.
-
Powerful Drosophila screens that paved the wingless pathway.Fly (Austin). 2014;8(4):218-25. doi: 10.4161/19336934.2014.985988. Epub 2015 Jan 20. Fly (Austin). 2014. PMID: 25565425 Free PMC article. Review.
Cited by
-
Carrier of Wingless (Cow) Regulation of Drosophila Neuromuscular Junction Development.eNeuro. 2020 Mar 10;7(2):ENEURO.0285-19.2020. doi: 10.1523/ENEURO.0285-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32024666 Free PMC article.
-
Evaluation of Human-Induced Pluripotent Stem Cells Derived from a Patient with Schwartz-Jampel Syndrome Revealed Distinct Hyperexcitability in the Skeletal Muscles.Biomedicines. 2023 Mar 7;11(3):814. doi: 10.3390/biomedicines11030814. Biomedicines. 2023. PMID: 36979792 Free PMC article.
-
Wnt Secretion Is Regulated by the Tetraspan Protein HIC-1 through Its Interaction with Neurabin/NAB-1.Cell Rep. 2018 Nov 13;25(7):1856-1871.e6. doi: 10.1016/j.celrep.2018.10.053. Cell Rep. 2018. PMID: 30428353 Free PMC article.
-
Functional analysis of glycosylation using Drosophila melanogaster.Glycoconj J. 2020 Feb;37(1):1-14. doi: 10.1007/s10719-019-09892-0. Epub 2019 Nov 26. Glycoconj J. 2020. PMID: 31773367 Review.
-
Heparan sulfate proteoglycans regulate autophagy in Drosophila.Autophagy. 2017 Aug 3;13(8):1262-1279. doi: 10.1080/15548627.2017.1304867. Epub 2017 Apr 12. Autophagy. 2017. PMID: 28402693 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

