Inhibition of triple-negative breast cancer models by combinations of antibodies to EGFR
- PMID: 23319610
- PMCID: PMC3562774
- DOI: 10.1073/pnas.1220763110
Inhibition of triple-negative breast cancer models by combinations of antibodies to EGFR
Abstract
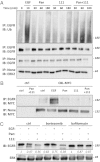
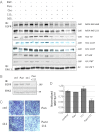
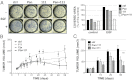
Breast tumors lacking expression of human epidermal growth factor receptor 2 (HER2) and the estrogen and the progesterone receptors (triple negative; TNBC) are more aggressive than other disease subtypes, and no molecular targeted agents are currently available for their treatment. Because TNBC commonly displays EGF receptor (EGFR) expression, and combinations of monoclonal antibodies to EGFR effectively inhibit other tumor models, we addressed the relevance of this strategy to treatment of TNBC. Unlike a combination of the clinically approved monoclonal antibodies, cetuximab and panitumumab, which displaced each other and displayed no cooperative effects, several other combinations resulted in enhanced inhibition of TNBC's cell growth both in vitro and in animals. The ability of certain antibody mixtures to remove EGFR from the cell surface and to promote its intracellular degradation correlated with the inhibitory potential. However, unlike EGF-induced sorting of EGFR to lysosomal degradation, the antibody-induced pathway displayed independence from the intrinsic kinase activity and dimer formation ability of EGFR, and it largely avoided the recycling route. In conclusion, although TNBC clinical trials testing EGFR inhibitors reported lack of benefit, our results offer an alternative strategy that combines noncompetitive antibodies to achieve robust degradation of EGFR and tumor inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Combinatorial effects of lapatinib and rapamycin in triple-negative breast cancer cells.Mol Cancer Ther. 2011 Aug;10(8):1460-9. doi: 10.1158/1535-7163.MCT-10-0925. Epub 2011 Jun 20. Mol Cancer Ther. 2011. PMID: 21690228 Free PMC article.
-
Growth and molecular interactions of the anti-EGFR antibody cetuximab and the DNA cross-linking agent cisplatin in gefitinib-resistant MDA-MB-468 cells: new prospects in the treatment of triple-negative/basal-like breast cancer.Int J Oncol. 2008 Dec;33(6):1165-76. Int J Oncol. 2008. PMID: 19020749
-
Nuclear epidermal growth factor receptor is a functional molecular target in triple-negative breast cancer.Mol Cancer Ther. 2014 May;13(5):1356-68. doi: 10.1158/1535-7163.MCT-13-1021. Epub 2014 Mar 14. Mol Cancer Ther. 2014. PMID: 24634415 Free PMC article.
-
Role of epidermal growth factor receptor in breast cancer.Breast Cancer Res Treat. 2012 Nov;136(2):331-45. doi: 10.1007/s10549-012-2289-9. Epub 2012 Oct 17. Breast Cancer Res Treat. 2012. PMID: 23073759 Free PMC article. Review.
-
Characteristics of triple-negative breast cancer.J Cancer Res Clin Oncol. 2011 Feb;137(2):183-92. doi: 10.1007/s00432-010-0957-x. Epub 2010 Nov 11. J Cancer Res Clin Oncol. 2011. PMID: 21069385 Free PMC article. Review.
Cited by
-
Diverse Roles of Annexin A6 in Triple-Negative Breast Cancer Diagnosis, Prognosis and EGFR-Targeted Therapies.Cells. 2020 Aug 7;9(8):1855. doi: 10.3390/cells9081855. Cells. 2020. PMID: 32784650 Free PMC article. Review.
-
Immunotherapeutic interventions of Triple Negative Breast Cancer.J Transl Med. 2018 May 30;16(1):147. doi: 10.1186/s12967-018-1514-7. J Transl Med. 2018. PMID: 29848327 Free PMC article. Review.
-
PCSK9 Promotes the Malignancy of Triple-negative Breast Cancer Cells by Reducing Cholesterol Levels at the Plasma Membrane to Activate EGFR and HER3.Adv Sci (Weinh). 2025 May;12(20):e2408514. doi: 10.1002/advs.202408514. Epub 2025 Apr 7. Adv Sci (Weinh). 2025. PMID: 40192514 Free PMC article.
-
Ultrasound-guided photoacoustic imaging for the selective detection of EGFR-expressing breast cancer and lymph node metastases.Biomed Opt Express. 2016 Apr 19;7(5):1920-31. doi: 10.1364/BOE.7.001920. eCollection 2016 May 1. Biomed Opt Express. 2016. PMID: 27231631 Free PMC article.
-
Endocytosis and cancer.Cold Spring Harb Perspect Biol. 2013 Dec 1;5(12):a016949. doi: 10.1101/cshperspect.a016949. Cold Spring Harb Perspect Biol. 2013. PMID: 24296170 Free PMC article. Review.
References
-
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. - PubMed
-
- Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2(3):273–277. - PubMed
-
- Spiridon CI, et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8(6):1720–1730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

