Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies
- PMID: 23319701
- PMCID: PMC3570107
- DOI: 10.1084/jem.20121970
Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies
Abstract
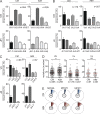
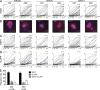
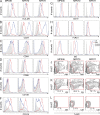
Antibodies can protect from Plasmodium falciparum (Pf) infection and clinical malaria disease. However, in the absence of constant reexposure, serum immunoglobulin (Ig) levels rapidly decline and full protection from clinical symptoms is lost, suggesting that B cell memory is functionally impaired. We show at the single cell level that natural Pf infection induces the development of classical memory B cells (CM) and atypical memory B cells (AtM) that produce broadly neutralizing antibodies against blood stage Pf parasites. CM and AtM contribute to anti-Pf serum IgG production, but only AtM show signs of active antibody secretion. AtM and CM were also different in their IgG gene repertoire, suggesting that they develop from different precursors. The findings provide direct evidence that natural Pf infection leads to the development of protective memory B cell antibody responses and suggest that constant immune activation rather than impaired memory function leads to the accumulation of AtM in malaria. Understanding the memory B cell response to natural Pf infection may be key to the development of a malaria vaccine that induces long-lived protection.
Figures




References
-
- Bouharoun-Tayoun H., Attanath P., Sabchareon A., Chongsuphajaisiddhi T., Druilhe P. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633–1641 10.1084/jem.172.6.1633 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

