Trp53 inactivation in the tumor microenvironment promotes tumor progression by expanding the immunosuppressive lymphoid-like stromal network
- PMID: 23319800
- PMCID: PMC3602383
- DOI: 10.1158/0008-5472.CAN-12-3810
Trp53 inactivation in the tumor microenvironment promotes tumor progression by expanding the immunosuppressive lymphoid-like stromal network
Abstract
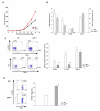
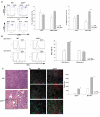
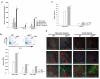
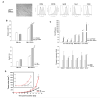
Inactivation of the tumor suppressor p53 through somatic mutations, observed in 50% of human cancers, is one of the leading causes of tumorigenesis. Clinical and experimental evidence also reveals that p53 mutations sometimes occur in tumor-associated fibroblasts, which correlate with an increased rate of metastases and poor prognosis, suggesting that p53 dysfunction in the tumor microenvironment (TME) favors tumor establishment and progression. To understand the impact of p53 inactivation in the TME in tumor progression, we compared the growth of subcutaneously inoculated B16F1 melanoma in p53(null) and wild-type (WT) mice. Interestingly, tumor growth in p53(null) mice was greatly accelerated, correlating with marked increases in CD11b(+)Gr-1(+) myeloid-derived suppressor cells (MDSC), FoxP3(+) regulatory T cells, and a loss of effector function, compared with those in WT mice. This augmented immunotolerant TME in p53(null) mice was associated with a marked expansion of a specialized stromal network in the tumor and spleen. These stromal cells expressed markers of fibroblastic reticular cells of lymphoid organs and were readily expanded in culture from p53(null), but not WT, mice. They produced high levels of inflammatory cytokines/chemokines and immunosuppressive molecules, thereby enhancing MDSC differentiation. Furthermore, they significantly accelerated tumor progression in WT mice when co-injected with B16F1. Together, our results show that tumor-stroma interaction in hosts with dysfunctional p53 exacerbates immunosuppression by expanding the lymphoid-like stromal network that enhances MDSC differentiation and tumor progression.
Figures
Similar articles
-
Direct interaction with and activation of p53 by SMAR1 retards cell-cycle progression at G2/M phase and delays tumor growth in mice.Int J Cancer. 2003 Feb 20;103(5):606-15. doi: 10.1002/ijc.10881. Int J Cancer. 2003. PMID: 12494467
-
Local Activation of p53 in the Tumor Microenvironment Overcomes Immune Suppression and Enhances Antitumor Immunity.Cancer Res. 2017 May 1;77(9):2292-2305. doi: 10.1158/0008-5472.CAN-16-2832. Epub 2017 Mar 9. Cancer Res. 2017. PMID: 28280037 Free PMC article.
-
Intestinal cancer progression by mutant p53 through the acquisition of invasiveness associated with complex glandular formation.Oncogene. 2017 Oct 19;36(42):5885-5896. doi: 10.1038/onc.2017.194. Epub 2017 Jun 19. Oncogene. 2017. PMID: 28628120 Free PMC article.
-
Immunomodulatory Function of the Tumor Suppressor p53 in Host Immune Response and the Tumor Microenvironment.Int J Mol Sci. 2016 Nov 19;17(11):1942. doi: 10.3390/ijms17111942. Int J Mol Sci. 2016. PMID: 27869779 Free PMC article. Review.
-
Myeloid-Derived Suppressor Cells in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1224:117-140. doi: 10.1007/978-3-030-35723-8_8. Adv Exp Med Biol. 2020. PMID: 32036608 Review.
Cited by
-
Influenza A Virus Infection Triggers Pyroptosis and Apoptosis of Respiratory Epithelial Cells through the Type I Interferon Signaling Pathway in a Mutually Exclusive Manner.J Virol. 2018 Jun 29;92(14):e00396-18. doi: 10.1128/JVI.00396-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743359 Free PMC article.
-
Systems Immunology Analysis Reveals an Immunomodulatory Effect of Snail-p53 Binding on Neutrophil- and T Cell-Mediated Immunity in KRAS Mutant Non-Small Cell Lung Cancer.Front Immunol. 2020 Dec 14;11:569671. doi: 10.3389/fimmu.2020.569671. eCollection 2020. Front Immunol. 2020. PMID: 33381110 Free PMC article.
-
p53: A player in the tumor microenvironment.Oncol Res. 2025 Mar 19;33(4):795-810. doi: 10.32604/or.2025.057317. eCollection 2025. Oncol Res. 2025. PMID: 40191727 Free PMC article. Review.
-
Evasion of anti-growth signaling: A key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds.Semin Cancer Biol. 2015 Dec;35 Suppl:S55-S77. doi: 10.1016/j.semcancer.2015.02.005. Epub 2015 Mar 6. Semin Cancer Biol. 2015. PMID: 25749195 Free PMC article. Review.
-
Potential functional variants in SMC2 and TP53 in the AURORA pathway genes and risk of pancreatic cancer.Carcinogenesis. 2019 Jun 10;40(4):521-528. doi: 10.1093/carcin/bgz029. Carcinogenesis. 2019. PMID: 30794721 Free PMC article.
References
-
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. - PubMed
-
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. - PubMed
-
- Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene. 2007;26:2145–2156. - PubMed
-
- Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

