Conditional Inactivation of Pten with EGFR Overexpression in Schwann Cells Models Sporadic MPNST
- PMID: 23319880
- PMCID: PMC3539440
- DOI: 10.1155/2012/620834
Conditional Inactivation of Pten with EGFR Overexpression in Schwann Cells Models Sporadic MPNST
Abstract
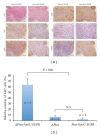
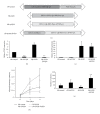
The genetic mechanisms involved in the transformation from a benign neurofibroma to a malignant sarcoma in patients with neurofibromatosis-type-1- (NF1-)associated or sporadic malignant peripheral nerve sheath tumors (MPNSTs) remain unclear. It is hypothesized that many genetic changes are involved in transformation. Recently, it has been shown that both phosphatase and tensin homolog (PTEN) and epidermal growth factor receptor (EGFR) play important roles in the initiation of peripheral nerve sheath tumors (PNSTs). In human MPNSTs, PTEN expression is often reduced, while EGFR expression is often induced. We tested if these two genes cooperate in the evolution of PNSTs. Transgenic mice were generated carrying conditional floxed alleles of Pten, and EGFR was expressed under the control of the 2',3'-cyclic nucleotide 3'phosphodiesterase (Cnp) promoter and a desert hedgehog (Dhh) regulatory element driving Cre recombinase transgenic mice (Dhh-Cre). Complete loss of Pten and EGFR overexpression in Schwann cells led to the development of high-grade PNSTs. In vitro experiments using immortalized human Schwann cells demonstrated that loss of PTEN and overexpression of EGFR cooperate to increase cellular proliferation and anchorage-independent colony formation. This mouse model can rapidly recapitulate PNST onset and progression to high-grade PNSTs, as seen in sporadic MPNST patients.
Figures



References
-
- Friedman JM. Epidemiology of neurofibromatosis type 1. American Journal of Medical Genetics. 1999;89(1):1–6. - PubMed
-
- Rosenfeld A, Listernick R, Charrow J, Goldman S. Neurofibromatosis type 1 and high-grade tumors of the central nervous system. Child’s Nervous System. 2010;26(5):663–667. - PubMed
-
- Beert E, Brems H, Daniels B, et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50(12):1021–1032. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

