Secreted factors from bone marrow stromal cells upregulate IL-10 and reverse acute kidney injury
- PMID: 23319959
- PMCID: PMC3539665
- DOI: 10.1155/2012/392050
Secreted factors from bone marrow stromal cells upregulate IL-10 and reverse acute kidney injury
Abstract
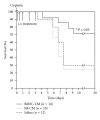
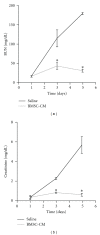
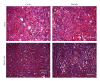
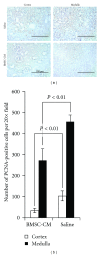
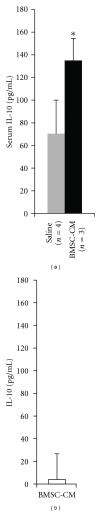
Acute kidney injury is a devastating syndrome that afflicts over 2,000,000 people in the US per year, with an associated mortality of greater than 70% in severe cases. Unfortunately, standard-of-care treatments are not sufficient for modifying the course of disease. Many groups have explored the use of bone marrow stromal cells (BMSCs) for the treatment of AKI because BMSCs have been shown to possess unique anti-inflammatory, cytoprotective, and regenerative properties in vitro and in vivo. It is yet unresolved whether the primary mechanisms controlling BMSC therapy in AKI depend on direct cell infusion, or whether BMSC-secreted factors alone are sufficient for mitigating the injury. Here we show that BMSC-secreted factors are capable of providing a survival benefit to rats subjected to cisplatin-induced AKI. We observed that when BMSC-conditioned medium (BMSC-CM) is administered intravenously, it prevents tubular apoptosis and necrosis and ameliorates AKI. In addition, we observed that BMSC-CM causes IL-10 upregulation in treated animals, which is important to animal survival and protection of the kidney. In all, these results demonstrate that BMSC-secreted factors are capable of providing support without cell transplantation, and the IL-10 increase seen in BMSC-CM-treated animals correlates with attenuation of severe AKI.
Figures





Similar articles
-
Paracrine Activation of the Wnt/β-Catenin Pathway by Bone Marrow Stem Cell Attenuates Cisplatin-Induced Kidney Injury.Cell Physiol Biochem. 2017;44(5):1980-1994. doi: 10.1159/000485904. Epub 2017 Dec 11. Cell Physiol Biochem. 2017. PMID: 29237155
-
miR-145 Regulates Diabetes-Bone Marrow Stromal Cell-Induced Neurorestorative Effects in Diabetes Stroke Rats.Stem Cells Transl Med. 2016 Dec;5(12):1656-1667. doi: 10.5966/sctm.2015-0349. Epub 2016 Jul 26. Stem Cells Transl Med. 2016. PMID: 27460851 Free PMC article.
-
Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment.Exp Cell Res. 2013 Aug 1;319(13):2019-2027. doi: 10.1016/j.yexcr.2013.04.008. Epub 2013 Apr 24. Exp Cell Res. 2013. PMID: 23624354
-
Therapeutic action of bone marrow-derived stem cells against acute kidney injury.Life Sci. 2014 Oct 12;115(1-2):1-7. doi: 10.1016/j.lfs.2014.08.025. Epub 2014 Sep 16. Life Sci. 2014. PMID: 25219881 Review.
-
Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro.Brain Res. 2019 Dec 15;1725:146432. doi: 10.1016/j.brainres.2019.146432. Epub 2019 Sep 3. Brain Res. 2019. PMID: 31491422 Review.
Cited by
-
Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models.Front Med (Lausanne). 2018 Jun 15;5:179. doi: 10.3389/fmed.2018.00179. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29963554 Free PMC article. Review.
-
Electric field-directed migration of mesenchymal stem cells enhances their therapeutic potential on cisplatin-induced acute nephrotoxicity in rats.Naunyn Schmiedebergs Arch Pharmacol. 2023 Jun;396(6):1077-1093. doi: 10.1007/s00210-022-02380-7. Epub 2023 Jan 14. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36640200 Free PMC article.
-
Extracorporeal Stromal Cell Therapy for Subjects With Dialysis-Dependent Acute Kidney Injury.Kidney Int Rep. 2018 Jun 2;3(5):1119-1127. doi: 10.1016/j.ekir.2018.05.009. eCollection 2018 Sep. Kidney Int Rep. 2018. PMID: 30197978 Free PMC article.
-
The role of indoleamine 2,3 dioxygenase in beneficial effects of stem cells in hind limb ischemia reperfusion injury.PLoS One. 2014 Apr 21;9(4):e95720. doi: 10.1371/journal.pone.0095720. eCollection 2014. PLoS One. 2014. PMID: 24752324 Free PMC article.
-
Extracellular vesicles derived from mesenchymal stromal cells may possess increased therapeutic potential for acute kidney injury compared with conditioned medium in rodent models: A meta-analysis.Exp Ther Med. 2016 Apr;11(4):1519-1525. doi: 10.3892/etm.2016.3076. Epub 2016 Feb 16. Exp Ther Med. 2016. PMID: 27073476 Free PMC article.
References
-
- Schrier RW, Wang W. Acute renal failure and sepsis. The New England Journal of Medicine. 2004;351(2):159–169. - PubMed
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology. 2005;16(11):3365–3370. - PubMed
-
- Bonventre JV. Mechanisms of acute kidney injury and repair. In: Jörres A, Ronco C, Kellum JA, editors. Management of Acute Kidney Problems. Berlin, Germany: Springer; 2010. pp. 13–20.
-
- Bonventre JV. Dialysis in acute kidney injury—more is not better. New England Journal of Medicine. 2008;359(1):82–84. - PubMed
-
- Brivet FG, Kleinknecht DJ, Loirat P, Landais PJM. Acute renal failure in intensive care units—causes, outcome, and prognostic factors of hospital mortality: a prospective, multicenter study. Critical Care Medicine. 1996;24(2):192–198. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

