Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms
- PMID: 23320034
- PMCID: PMC3539716
- DOI: 10.1155/2012/639469
Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms
Abstract
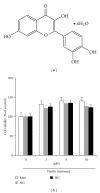
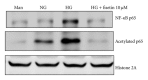
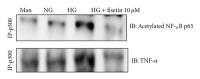
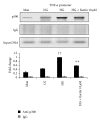
Diabetes is characterized by a proinflammatory state, and several inflammatory processes have been associated with both type 1 and type 2 diabetes and the resulting complications. High glucose levels induce the release of proinflammatory cytokines. Fisetin, a flavonoid dietary ingredient found in the smoke tree (Cotinus coggygria), and is also widely distributed in fruits and vegetables. Fisetin is known to exert anti-inflammatory effects via inhibition of the NF-κB signaling pathway. In this study, we analyzed the effects of fisetin on proinflammatory cytokine secretion and epigenetic regulation, in human monocytes cultured under hyperglycemic conditions. Human monocytic (THP-1) cells were cultured under control (14.5 mmol/L mannitol), normoglycemic (NG, 5.5 mmol/L glucose), or hyperglycemic (HG, 20 mmol/L glucose) conditions, in the absence or presence of fisetin. Fisetin was added (3-10 μM) for 48 h. While the HG condition significantly induced histone acetylation, NF-κB activation, and proinflammatory cytokine (IL-6 and TNF-α) release from THP-1 cells, fisetin suppressed NF-κB activity and cytokine release. Fisetin treatment also significantly reduced CBP/p300 gene expression, as well as the levels of acetylation and HAT activity of the CBP/p300 protein, which is a known NF-κB coactivator. These results suggest that fisetin inhibits HG-induced cytokine production in monocytes, through epigenetic changes involving NF-κB. We therefore propose that fisetin supplementation be considered for diabetes prevention.
Figures


Similar articles
-
Luteolin inhibits hyperglycemia-induced proinflammatory cytokine production and its epigenetic mechanism in human monocytes.Phytother Res. 2014 Sep;28(9):1383-91. doi: 10.1002/ptr.5141. Epub 2014 Mar 12. Phytother Res. 2014. PMID: 24623679
-
Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin.J Nutr Biochem. 2011 May;22(5):450-8. doi: 10.1016/j.jnutbio.2010.03.014. Epub 2010 Jul 22. J Nutr Biochem. 2011. PMID: 20655188 Free PMC article.
-
Combination Treatments with Luteolin and Fisetin Enhance Anti-Inflammatory Effects in High Glucose-Treated THP-1 Cells Through Histone Acetyltransferase/Histone Deacetylase Regulation.J Med Food. 2017 Aug;20(8):782-789. doi: 10.1089/jmf.2017.3968. Epub 2017 Jun 26. J Med Food. 2017. PMID: 28650731
-
Exploring the therapeutic promise of fisetin: molecular mechanisms and clinical aspects in lung cancer.J Complement Integr Med. 2025 Feb 28. doi: 10.1515/jcim-2024-0444. Online ahead of print. J Complement Integr Med. 2025. PMID: 40013371 Review.
-
An Overview of Recent Advances in the Neuroprotective Potentials of Fisetin against Diverse Insults in Neurological Diseases and the Underlying Signaling Pathways.Biomedicines. 2023 Oct 24;11(11):2878. doi: 10.3390/biomedicines11112878. Biomedicines. 2023. PMID: 38001882 Free PMC article. Review.
Cited by
-
Hyperglycemia promotes K-Ras-induced lung tumorigenesis through BASCs amplification.PLoS One. 2014 Aug 21;9(8):e105550. doi: 10.1371/journal.pone.0105550. eCollection 2014. PLoS One. 2014. PMID: 25144301 Free PMC article.
-
Molecular Mechanistic Pathways Targeted by Natural Compounds in the Prevention and Treatment of Diabetic Kidney Disease.Molecules. 2022 Sep 21;27(19):6221. doi: 10.3390/molecules27196221. Molecules. 2022. PMID: 36234757 Free PMC article. Review.
-
Adipose tissue inflammation linked to obesity: A review of current understanding, therapies and relevance of phyto-therapeutics.Heliyon. 2023 Dec 2;10(1):e23114. doi: 10.1016/j.heliyon.2023.e23114. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38163110 Free PMC article. Review.
-
The Role of Dietary Phenolic Compounds in Epigenetic Modulation Involved in Inflammatory Processes.Antioxidants (Basel). 2020 Aug 3;9(8):691. doi: 10.3390/antiox9080691. Antioxidants (Basel). 2020. PMID: 32756302 Free PMC article. Review.
-
Therapeutic perspectives of epigenetically active nutrients.Br J Pharmacol. 2015 Jun;172(11):2756-68. doi: 10.1111/bph.12854. Epub 2014 Dec 15. Br J Pharmacol. 2015. PMID: 25046997 Free PMC article. Review.
References
-
- Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectrum. 2008;21(3):160–165.
-
- Pugliese G, Tilton RG, Williamson JR. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes/Metabolism Reviews. 1991;7(1):35–59. - PubMed
-
- Ruderman N, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. The FASEB Journal. 1992;6:2905–2914. - PubMed
-
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. - PubMed
-
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55(3):774–779. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

