Caffeic Acid phenethyl ester inhibits oral cancer cell metastasis by regulating matrix metalloproteinase-2 and the mitogen-activated protein kinase pathway
- PMID: 23320037
- PMCID: PMC3535744
- DOI: 10.1155/2012/732578
Caffeic Acid phenethyl ester inhibits oral cancer cell metastasis by regulating matrix metalloproteinase-2 and the mitogen-activated protein kinase pathway
Erratum in
-
Corrigendum to "Caffeic Acid Phenethyl Ester Inhibits Oral Cancer Cell Metastasis by Regulating Matrix Metalloproteinase-2 and the Mitogen-Activated Protein Kinase Pathway".Evid Based Complement Alternat Med. 2016;2016:6728642. doi: 10.1155/2016/6728642. Epub 2016 Jun 28. Evid Based Complement Alternat Med. 2016. PMID: 27433184 Free PMC article.
Abstract
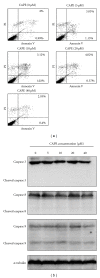
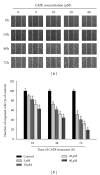
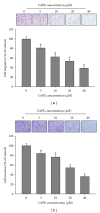
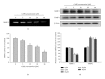
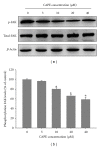
Caffeic acid phenethyl ester (CAPE), an active component extracted from honeybee hives, exhibits anti-inflammatory and anticancer activities. However, the molecular mechanism by which CAPE affects oral cancer cell metastasis has yet to be elucidated. In this study, we investigated the potential mechanisms underlying the effects of CAPE on the invasive ability of SCC-9 oral cancer cells. Results showed that CAPE attenuated SCC-9 cell migration and invasion at noncytotoxic concentrations (0 μM to 40 μM). Western blot and gelatin zymography analysis findings further indicated that CAPE downregulated matrix metalloproteinase-2 (MMP-2) protein expression and inhibited its enzymatic activity. CAPE exerted its inhibitory effects on MMP-2 expression and activity by upregulating tissue inhibitor of metalloproteinase-2 (TIMP-2) and potently decreased migration by reducing focal adhesion kinase (FAK) phosphorylation and the activation of its downstream signaling molecules p38/MAPK and JNK. These data indicate that CAPE could potentially be used as a chemoagent to prevent oral cancer metastasis.
Figures


References
-
- Spiro RH, Alfonso AE, Farr HW, Strong EW. Cervical node metastasis from epidermoid carcinoma of the oral cavity and oropharynx. A critical assessment of current staging. American Journal of Surgery. 1974;128(4):562–567. - PubMed
-
- Stetler-Stevenson WG, Liotta LA, Kleiner DE. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB Journal. 1993;7(15):1434–1441. - PubMed
-
- Chien MH, Ying TH, Hsieh YS, et al. Dioscorea nipponica Makino inhibits migration and invasion of human oral cancer HSC-3 cells by transcriptional inhibition of matrix metalloproteinase-2 through modulation of CREB and AP-1 activity. Food and Chemical Toxicology. 2012;50:558–566. - PubMed
-
- Yang SF, Chen MK, Hsieh YS, et al. Antimetastatic effects of Terminalia catappa L. on oral cancer via a down-regulation of metastasis-associated proteases. Food and Chemical Toxicology. 2010;48(4):1052–1058. - PubMed
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer. 2002;2(3):161–174. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

