Alternative Pathways of Cancer Cell Death by Rottlerin: Apoptosis versus Autophagy
- PMID: 23320042
- PMCID: PMC3541534
- DOI: 10.1155/2012/980658
Alternative Pathways of Cancer Cell Death by Rottlerin: Apoptosis versus Autophagy
Abstract
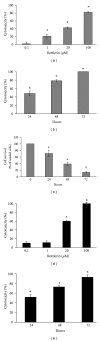
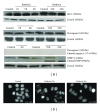
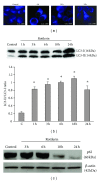
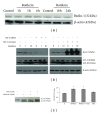
Since the ability of cancer cells to evade apoptosis often limits the efficacy of radiotherapy and chemotherapy, autophagy is emerging as an alternative target to promote cell death. Therefore, we wondered whether Rottlerin, a natural polyphenolic compound with antiproliferative effects in several cell types, can induce cell death in MCF-7 breast cancer cells. The MCF-7 cell line is a good model of chemo/radio resistance, being both apoptosis and autophagy resistant, due to deletion of caspase 3 gene, high expression of the antiapoptotic protein Bcl-2, and low expression of the autophagic Beclin-1 protein. The contribution of autophagy and apoptosis to the cytotoxic effects of Rottlerin was examined by light, fluorescence, and electron microscopic examination and by western blotting analysis of apoptotic and autophagic markers. By comparing caspases-3-deficient (MCF-7(3def)) and caspases-3-transfected MCF-7 cells (MCF-7(3trans)), we found that Rottlerin induced a noncanonical, Bcl-2-, Beclin 1-, Akt-, and ERK-independent autophagic death in the former- and the caspases-mediated apoptosis in the latter, in not starved conditions and in the absence of any other treatment. These findings suggest that Rottlerin could be cytotoxic for different cancer cell types, both apoptosis competent and apoptosis resistant.
Figures

Similar articles
-
Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells.Int J Med Sci. 2012;9(10):881-93. doi: 10.7150/ijms.5011. Epub 2012 Nov 7. Int J Med Sci. 2012. PMID: 23155362 Free PMC article.
-
A new synthetic HDAC inhibitor, MHY218, induces apoptosis or autophagy-related cell death in tamoxifen-resistant MCF-7 breast cancer cells.Invest New Drugs. 2012 Oct;30(5):1887-98. doi: 10.1007/s10637-011-9752-z. Epub 2011 Oct 8. Invest New Drugs. 2012. PMID: 21983700
-
Phosphorylation-independent mTORC1 inhibition by the autophagy inducer Rottlerin.Cancer Lett. 2015 Apr 28;360(1):17-27. doi: 10.1016/j.canlet.2015.01.040. Epub 2015 Feb 4. Cancer Lett. 2015. PMID: 25661734
-
Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer.Cell Death Dis. 2014 Nov 6;5(11):e1509. doi: 10.1038/cddis.2014.467. Cell Death Dis. 2014. PMID: 25375374 Free PMC article. Review.
-
Tumor suppressive role of rottlerin in cancer therapy.Am J Transl Res. 2018 Nov 15;10(11):3345-3356. eCollection 2018. Am J Transl Res. 2018. PMID: 30662591 Free PMC article. Review.
Cited by
-
New Visions on Natural Products and Cancer Therapy: Autophagy and Related Regulatory Pathways.Cancers (Basel). 2022 Nov 26;14(23):5839. doi: 10.3390/cancers14235839. Cancers (Basel). 2022. PMID: 36497321 Free PMC article. Review.
-
Rottlerin promotes autophagy and apoptosis in gastric cancer cell lines.Mol Med Rep. 2018 Sep;18(3):2905-2913. doi: 10.3892/mmr.2018.9293. Epub 2018 Jul 16. Mol Med Rep. 2018. PMID: 30015872 Free PMC article.
-
Toll-like receptor 9 activation by CpG oligodeoxynucleotide 7909 enhances the radiosensitivity of A549 lung cancer cells via the p53 signaling pathway.Oncol Lett. 2018 Apr;15(4):5271-5279. doi: 10.3892/ol.2018.7916. Epub 2018 Jan 31. Oncol Lett. 2018. PMID: 29541253 Free PMC article.
-
Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells.Sci Rep. 2014 Jul 1;4:5510. doi: 10.1038/srep05510. Sci Rep. 2014. PMID: 24981420 Free PMC article.
-
Rottlerin-mediated inhibition of Toxoplasma gondii growth in BeWo trophoblast-like cells.Sci Rep. 2017 Apr 28;7(1):1279. doi: 10.1038/s41598-017-01525-6. Sci Rep. 2017. PMID: 28455500 Free PMC article.
References
-
- Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resistance Updates. 2007;10(4-5):135–143. - PubMed
-
- Patel VR, Patel MG, Patel RK. Development and validation of a RP-HPLC method for quantification of rottlerin in Kamala (Mallotus philppinensis) Drug Invention Today. 2009;1(2):116–118.
-
- Torricelli C, Fortino V, Capurro E, et al. Rottlerin inhibits the nuclear factor κB/Cyclin-D1 cascade in MCF-7 breast cancer cells. Life Sciences. 2008;82(11-12):638–643. - PubMed
-
- Valacchi G, Pecorelli A, Mencarelli M, et al. Rottlerin: a multifaced regulator of keratinocyte cell cycle. Experimental Dermatology. 2009;18(6):516–521. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

