Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation
- PMID: 23320049
- PMCID: PMC3539293
- DOI: 10.1177/1756283X12459294
Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation
Abstract
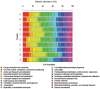
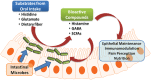
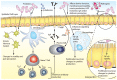
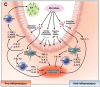
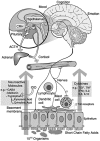
Recent explorations of the human gut microbiota suggest that perturbations of microbial communities may increase predisposition to different disease phenotypes. Dietary nutrients may be converted into metabolites by intestinal microbes that serve as biologically active molecules affecting regulatory functions in the host. Probiotics may restore the composition of the gut microbiome and introduce beneficial functions to gut microbial communities, resulting in amelioration or prevention of gut inflammation and other intestinal or systemic disease phenotypes. This review describes how diet and intestinal luminal conversion by gut microbes play a role in shaping the structure and function of intestinal microbial communities. Proposed mechanisms of probiosis include alterations of composition and function of the human gut microbiome, and corresponding effects on immunity and neurobiology.
Keywords: Lactobacillus; diet; gut microbiota; immunomodulation; nervous system; probiotics.
Conflict of interest statement
Figures
Similar articles
-
Effects of Probiotics on Gut Microbiota: An Overview.Int J Mol Sci. 2024 May 30;25(11):6022. doi: 10.3390/ijms25116022. Int J Mol Sci. 2024. PMID: 38892208 Free PMC article. Review.
-
Luminal Conversion and Immunoregulation by Probiotics.Front Pharmacol. 2015 Nov 12;6:269. doi: 10.3389/fphar.2015.00269. eCollection 2015. Front Pharmacol. 2015. PMID: 26617521 Free PMC article. Review.
-
Microbial communities modulating brain functioning and behaviors in zebrafish: A mechanistic approach.Microb Pathog. 2020 Aug;145:104251. doi: 10.1016/j.micpath.2020.104251. Epub 2020 May 11. Microb Pathog. 2020. PMID: 32418919 Review.
-
Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex.J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008. Epub 2018 Jun 1. J Autoimmun. 2018. PMID: 29861127 Review.
-
The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome.Curr Med Chem. 2019;26(19):3567-3583. doi: 10.2174/0929867324666170428103848. Curr Med Chem. 2019. PMID: 28462705 Review.
Cited by
-
Coronavirus disease-2019 and the intestinal tract: An overview.World J Gastroenterol. 2021 Apr 7;27(13):1255-1266. doi: 10.3748/wjg.v27.i13.1255. World J Gastroenterol. 2021. PMID: 33833480 Free PMC article. Review.
-
Dietary Factors Associated with Asthma Development: A Narrative Review and Summary of Current Guidelines and Recommendations.J Asthma Allergy. 2022 Aug 24;15:1125-1141. doi: 10.2147/JAA.S364964. eCollection 2022. J Asthma Allergy. 2022. PMID: 36046721 Free PMC article. Review.
-
Effect of Multi-Strain Probiotic Supplementation on URTI Symptoms and Cytokine Production by Monocytes after a Marathon Race: A Randomized, Double-Blind, Placebo Study.Nutrients. 2021 Apr 27;13(5):1478. doi: 10.3390/nu13051478. Nutrients. 2021. PMID: 33925633 Free PMC article. Clinical Trial.
-
Gastrointestinal dysfunction in Parkinson's disease: molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation.J Neurol. 2022 Mar;269(3):1154-1163. doi: 10.1007/s00415-021-10567-w. Epub 2021 Apr 21. J Neurol. 2022. PMID: 33881598 Review.
-
Gastrointestinal microbiota profile and clinical correlations in advanced EGFR-WT and EGFR-mutant non-small cell lung cancer.BMC Cancer. 2022 Sep 8;22(1):963. doi: 10.1186/s12885-022-10050-3. BMC Cancer. 2022. PMID: 36076157 Free PMC article.
References
-
- Artis D. (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8: 411–420 - PubMed
-
- Berer K., Mues M., Koutrolos M., Rasbi Z., Boziki M., Johner C., et al. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479: 538–541 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

