Does changing androgen receptor status during prostate cancer development impact upon cholesterol homeostasis?
- PMID: 23320115
- PMCID: PMC3540066
- DOI: 10.1371/journal.pone.0054007
Does changing androgen receptor status during prostate cancer development impact upon cholesterol homeostasis?
Abstract
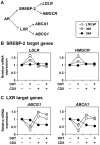
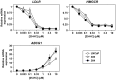
Background: Recent evidence associates prostate cancer with high cholesterol levels, with cholesterol being an important raw material for cell-growth. Within the cell, cholesterol homeostasis is maintained by two master transcription factors: sterol-regulatory element-binding protein 2 (SREBP-2) and liver X receptor (LXR). We previously showed that the androgen receptor, a major player in prostate cell physiology, toggles these transcription factors to promote cholesterol accumulation. Given that prostate cancer therapy targets the androgen receptor, selecting for cells with altered androgen receptor activity, how would this affect SREBP-2 and LXR activity? Using a novel prostate cancer progression model, we explored how this crosstalk between the androgen receptor and cholesterol homeostasis changes during prostate cancer development.
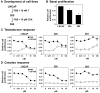
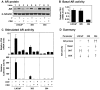
Methodology/principal findings: Firstly, we characterised our progression model, which involved 1) culturing LNCaP cells at physiological testosterone levels to generate androgen-tolerant LNCaP-305 cells, and 2) culturing LNCaP-305 with the anti-androgen casodex to generate castration-resistant LNCaP-364 cells. This progression was accompanied by upregulated androgen receptor expression, typically seen clinically, and a reduction in androgen receptor activity. Although this influenced how SREBP-2 and LXR target genes responded to androgen treatment, cellular cholesterol levels and their response to changing sterol status was similar in all LNCaP sub-lines.
Conclusion/significance: Overall cholesterol homeostasis is unaffected by changing androgen receptor activity in prostate cancer cells. This does not negate the relationship between androgens and cholesterol homeostasis, but rather suggests that other factors compensate for altered androgen receptor activity. Given that cholesterol regulation is maintained during progression, this supports the growing idea that cholesterol metabolism is a suitable target for prostate cancer.
Conflict of interest statement
Figures


References
-
- Huggins C, Hodges CV (1941) Studies on prostatic cancer – I The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1: 293–297. - PubMed
-
- Olsson M, Ekstrom L, Guillemette C, Belanger A, Rane A, et al. (2011) Correlation between circulatory, local prostatic, and intra-prostatic androgen levels. Prostate 71: 909–914. - PubMed
-
- Akaza H (2011) Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci 102: 51–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

