DNA methyltransferase inhibitor zebularine inhibits human hepatic carcinoma cells proliferation and induces apoptosis
- PMID: 23320119
- PMCID: PMC3540068
- DOI: 10.1371/journal.pone.0054036
DNA methyltransferase inhibitor zebularine inhibits human hepatic carcinoma cells proliferation and induces apoptosis
Abstract
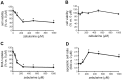
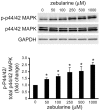
Hepatocellular carcinoma is one of the most common cancers worldwide. During tumorigenesis, tumor suppressor and cancer-related genes are commonly silenced by aberrant DNA methylation in their promoter regions. Zebularine (1-(β-(D)-ribofuranosyl)-1,2-dihydropyrimidin-2-one) acts as an inhibitor of DNA methylation and exhibits chemical stability and minimal cytotoxicity both in vitro and in vivo. In this study, we explore the effect and possible mechanism of action of zebularine on hepatocellular carcinoma cell line HepG2. We demonstrate that zebularine exhibits antitumor activity on HepG2 cells by inhibiting cell proliferation and inducing apoptosis, however, it has little effect on DNA methylation in HepG2 cells. On the other hand, zebularine treatment downregulated CDK2 and the phosphorylation of retinoblastoma protein (Rb), and upregulated p21(WAF/CIP1) and p53. We also found that zebularine treatment upregulated the phosphorylation of p44/42 mitogen-activated protein kinase (MAPK). These results suggest that the p44/42 MAPK pathway plays a role in zebularine-induced cell-cycle arrest by regulating the activity of p21(WAF/CIP1) and Rb. Furthermore, although the proapoptotic protein Bax levels were not affected, the antiapoptotic protein Bcl-2 level was downregulated with zebularine treatment. In addition, the data in the present study indicate that inhibition of the double-stranded RNA-dependent protein kinase (PKR) is involved in inducing apoptosis with zebularine. These results suggest a novel mechanism of zebularine-induced cell growth arrest and apoptosis via a DNA methylation-independent pathway in hepatocellular carcinoma.
Conflict of interest statement
Figures






Similar articles
-
DNA methyltransferase inhibitor zebularine induces human cholangiocarcinoma cell death through alteration of DNA methylation status.PLoS One. 2015 Mar 23;10(3):e0120545. doi: 10.1371/journal.pone.0120545. eCollection 2015. PLoS One. 2015. PMID: 25799509 Free PMC article.
-
Effects of a novel DNA methyltransferase inhibitor Zebularine on human lens epithelial cells.Mol Vis. 2012;18:22-8. Epub 2012 Jan 10. Mol Vis. 2012. PMID: 22259221 Free PMC article.
-
A novel anticancer agent, SKLB70359, inhibits human hepatic carcinoma cells proliferation via G0/G1 cell cycle arrest and apoptosis induction.Cell Physiol Biochem. 2012;29(1-2):281-90. doi: 10.1159/000337609. Epub 2012 Mar 1. Cell Physiol Biochem. 2012. PMID: 22415097
-
Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy.Ann N Y Acad Sci. 2005 Nov;1058:246-54. doi: 10.1196/annals.1359.037. Ann N Y Acad Sci. 2005. PMID: 16394141 Review.
-
Cytidine analogs in plant epigenetic research and beyond.J Exp Bot. 2025 Jun 17;76(9):2419-2432. doi: 10.1093/jxb/erae522. J Exp Bot. 2025. PMID: 39731754 Review.
Cited by
-
Epigenetics in hepatocellular carcinoma: an update and future therapy perspectives.World J Gastroenterol. 2014 Jan 14;20(2):333-45. doi: 10.3748/wjg.v20.i2.333. World J Gastroenterol. 2014. PMID: 24574704 Free PMC article. Review.
-
Genome-wide methylation study on depression: differential methylation and variable methylation in monozygotic twins.Transl Psychiatry. 2015 Apr 28;5(4):e557. doi: 10.1038/tp.2015.49. Transl Psychiatry. 2015. PMID: 25918994 Free PMC article.
-
Epigenetic regulation in hepatocellular carcinoma requires long noncoding RNAs.Biomed Res Int. 2015;2015:473942. doi: 10.1155/2015/473942. Epub 2015 Mar 10. Biomed Res Int. 2015. PMID: 25861629 Free PMC article. Review.
-
Epigenetic variability in conversion to psychosis: novel findings from an innovative longitudinal methylomic analysis.Transl Psychiatry. 2018 Apr 26;8(1):93. doi: 10.1038/s41398-018-0138-2. Transl Psychiatry. 2018. PMID: 29695761 Free PMC article.
-
Investigation of the Effect of Zebularine in Comparison to and in Combination with Trichostatin A on p21Cip1/Waf1/ Sdi1, p27Kip1, p57Kip2, DNA Methyltransferases and Histone Deacetylases in Colon Cancer LS 180 Cell Line.Asian Pac J Cancer Prev. 2020 Jun 1;21(6):1819-1828. doi: 10.31557/APJCP.2020.21.6.1819. Asian Pac J Cancer Prev. 2020. PMID: 32592383 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

