Ultrastructure of calcareous dinophytes (Thoracosphaeraceae, Peridiniales) with a focus on vacuolar crystal-like particles
- PMID: 23320120
- PMCID: PMC3540067
- DOI: 10.1371/journal.pone.0054038
Ultrastructure of calcareous dinophytes (Thoracosphaeraceae, Peridiniales) with a focus on vacuolar crystal-like particles
Abstract
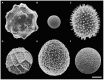
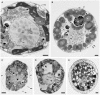
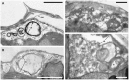
Biomineralization in calcareous dinophytes (Thoracosphaeracaea, Peridiniales) takes place in coccoid cells and is presently poorly understood. Vacuolar crystal-like particles as well as collection sites within the prospective calcareous shell may play a crucial role during this process at the ultrastructural level. Using transmission electron microscopy, we investigated the ultrastructure of coccoid cells at an early developmental stage in fourteen calcareous dinophyte strains (corresponding to at least ten species of Calciodinellum, Calcigonellum, Leonella, Pernambugia, Scrippsiella, and Thoracosphaera). The shell of the coccoid cells consisted either of one (Leonella, Thoracosphaera) or two matrices (Scrippsiella and its relatives) of unknown element composition, whereas calcite is deposited in the only or the outer layer, respectively. We observed crystal-like particles in cytoplasmic vacuoles in cells of nine of the strains investigated and assume that they are widespread among calcareous dinophytes. However, similar structures are also found outside the Thoracosphaeraceae, and we postulate an evolutionarily old physiological pathway (possibly involved in detoxification) that later was specialized for calcification. We aim to contribute to a deeper knowledge of the biomineralization process in calcareous dinophytes.
Conflict of interest statement
Figures



Similar articles
-
SAME BUT DIFFERENT: TWO NOVEL BICARINATE SPECIES OF EXTANT CALCAREOUS DINOPHYTES (THORACOSPHAERACEAE, PERIDINIALES) FROM THE MEDITERRANEAN SEA(1).J Phycol. 2012 Oct;48(5):1107-18. doi: 10.1111/j.1529-8817.2012.01182.x. Epub 2012 Jun 21. J Phycol. 2012. PMID: 27011272
-
Delimitation of the Thoracosphaeraceae (Dinophyceae), including the calcareous dinoflagellates, based on large amounts of ribosomal RNA sequence data.Protist. 2012 Jan;163(1):15-24. doi: 10.1016/j.protis.2011.06.003. Epub 2011 Jul 8. Protist. 2012. PMID: 21741879
-
TIMING DEEP DIVERGENCE EVENTS IN CALCAREOUS DINOFLAGELLATES(1).J Phycol. 2008 Apr;44(2):429-38. doi: 10.1111/j.1529-8817.2008.00479.x. J Phycol. 2008. PMID: 27041198
-
An Updated List of Generic Names in the Thoracosphaeraceae.Microorganisms. 2013 Nov 1;1(1):122-136. doi: 10.3390/microorganisms1010122. Microorganisms. 2013. PMID: 27694767 Free PMC article. Review.
-
Structure and composition of calcareous sponge spicules: a review and comparison to structurally related biominerals.Micron. 2008;39(3):209-28. doi: 10.1016/j.micron.2007.01.006. Epub 2007 Feb 3. Micron. 2008. PMID: 17360189 Review.
Cited by
-
Differences in Specific Mass Density Between Dinoflagellate Life Stages and Relevance to Accumulation by Hydrodynamic Processes.J Phycol. 2021 Oct;57(5):1492-1503. doi: 10.1111/jpy.13181. Epub 2021 Jun 17. J Phycol. 2021. PMID: 33960400 Free PMC article.
References
-
- De Yoreo JJ, Vekilov PG (2003) Principles of crystal nucleation and growth. Rev Mineral Geochem 54: 57–94.
-
- Knoll AH (2003) Biomineralization and evolutionary history. In: Dove PM, De Yoreo JJ, Weiner S, editors. Biomineralization. Washington, DC: Mineralogical Society of America. pp 329–356.
-
- Murdock DJE, Donoghue PCJ (2011) Evolutionary origins of animal skeletal biomineralization. Cells Tissues Organs 194: 98–102. - PubMed
-
- Raven JA, Giordano M (2009) Biomineralization by photosynthetic organisms: Evidence of coevolution of the organisms and their environment? Geobiology 7: 140–154. - PubMed
-
- Bäuerlein E (2004) Biomineralization. Progress in biology, molecular biology and application. Weinheim: Wiley.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

