Biocompatibility of intracanal medications based on calcium hydroxide
- PMID: 23320187
- PMCID: PMC3535743
- DOI: 10.5402/2012/904963
Biocompatibility of intracanal medications based on calcium hydroxide
Abstract
Objective. The aim of this study was to evaluate the rat subcutaneous tissue reaction to calcium hydroxide-based intracanal medicaments, UltraCal XS (calcium hydroxide, barium sulphate, aqueous matrix), Hydropast (calcium hydroxide, barium sulphate, and propyleneglycol), and Calen (Calcium hydroxide, zinc oxide, colophony, and polyethyleneglycol), used as a control. Methods. Forty-eight rats (Rattus Norvegicus Holtzman) were distributed in three groups: Calen, UltraCal XS, and Hydropast. Polyethylene tubes filled with one of the medicaments were implanted in the dorsal subcutaneous. After 7 and 30 days, the implants were removed and the specimens were fixed and embedded in paraffin. Morphological and quantitative analyses were carried out in the HE-stained sections. The numerical density of inflammatory cells in the capsule was evaluated and statistical analyses were performed (P ≤ 0.05). Results. At 7 days, all materials induced an inflammatory reaction in the subcutaneous tissue adjacent to the implants. In all groups, a significant reduction in the number of inflammatory cells and giant cells was verified in the period of 30 days. Conclusion. These results indicate that the calcium hydroxide-based medicaments evaluated present biocompatibility similar to Calen.
Figures

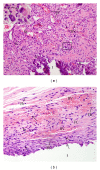
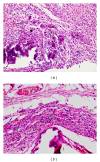
References
-
- Tronstad L. Recent development in endodontic research. Scandinavian Journal of Dental Research. 1992;100(1):52–59. - PubMed
-
- Siqueira JF., Jr. Aetiology of root canal treatment failure: why well-treated teeth can fail. International Endodontic Journal. 2001;34(1):1–10. - PubMed
-
- Faria G, Nelson-Filho P, Freitas AC, Assed S, Ito IY. Antibacterial effect of root canal preparation and calcium hydroxide paste (Calen) intracanal dressing in primary teeth with apical periodontitis. Journal of Applied Oral Science. 2005;13:351–355. - PubMed
-
- Leonardo MR, Hernandez MEFT, Silva LAB, Tanomaru-Filho M. Effect of a calcium hydroxide-based root canal dressing on periapical repair in dogs: a histological study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2006;102(5):680–685. - PubMed
-
- Lima RKP, Guerreiro-Tanomaru JM, Faria-Júnior NB, Tanomaru-Filho M. Effectiveness of calcium hydroxide-based intracanal medicaments against Enterococcus faecalis. International Endodontic Journal. 2012;45(4):311–316. - PubMed
LinkOut - more resources
Full Text Sources

