Modulation of gamma-secretase for the treatment of Alzheimer's disease
- PMID: 23320246
- PMCID: PMC3536039
- DOI: 10.1155/2012/210756
Modulation of gamma-secretase for the treatment of Alzheimer's disease
Abstract
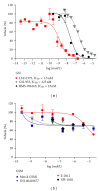
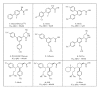
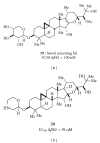
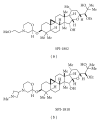
The Amyloid Hypothesis states that the cascade of events associated with Alzheimer's disease (AD)-formation of amyloid plaques, neurofibrillary tangles, synaptic loss, neurodegeneration, and cognitive decline-are triggered by Aβ peptide dysregulation (Kakuda et al., 2006, Sato et al., 2003, Qi-Takahara et al., 2005). Since γ-secretase is critical for Aβ production, many in the biopharmaceutical community focused on γ-secretase as a target for therapeutic approaches for Alzheimer's disease. However, pharmacological approaches to control γ-secretase activity are challenging because the enzyme has multiple, physiologically critical protein substrates. To lower amyloidogenic Aβ peptides without affecting other γ-secretase substrates, the epsilon (ε) cleavage that is essential for the activity of many substrates must be preserved. Small molecule modulators of γ-secretase activity have been discovered that spare the ε cleavage of APP and other substrates while decreasing the production of Aβ(42). Multiple chemical classes of γ-secretase modulators have been identified which differ in the pattern of Aβ peptides produced. Ideally, modulators will allow the ε cleavage of all substrates while shifting APP cleavage from Aβ(42) and other highly amyloidogenic Aβ peptides to shorter and less neurotoxic forms of the peptides without altering the total Aβ pool. Here, we compare chemically distinct modulators for effects on APP processing and in vivo activity.
Figures


Similar articles
-
Orally bioavailable and brain-penetrant pyridazine and pyridine-derived γ-secretase modulators reduced amyloidogenic Aβ peptides in vivo.Neuropharmacology. 2013 Jul;70:278-86. doi: 10.1016/j.neuropharm.2013.02.003. Epub 2013 Feb 26. Neuropharmacology. 2013. PMID: 23485401
-
BIIB042, a novel γ-secretase modulator, reduces amyloidogenic Aβ isoforms in primates and rodents and plaque pathology in a mouse model of Alzheimer's disease.Neuropharmacology. 2016 Apr;103:57-68. doi: 10.1016/j.neuropharm.2015.12.006. Epub 2015 Dec 12. Neuropharmacology. 2016. PMID: 26690893
-
Ibuprofen and lipoic acid codrug 1 control Alzheimer's disease progression by down-regulating protein kinase C ε-mediated metalloproteinase 2 and 9 levels in β-amyloid infused Alzheimer's disease rat model.Brain Res. 2011 Sep 15;1412:79-87. doi: 10.1016/j.brainres.2011.07.022. Epub 2011 Jul 20. Brain Res. 2011. PMID: 21820649
-
Inhibition of gamma-secretase as a therapeutic intervention for Alzheimer's disease: prospects, limitations and strategies.CNS Drugs. 2006;20(5):351-72. doi: 10.2165/00023210-200620050-00002. CNS Drugs. 2006. PMID: 16696577 Review.
-
Targeting Amyloidogenic Processing of APP in Alzheimer's Disease.Front Mol Neurosci. 2020 Aug 4;13:137. doi: 10.3389/fnmol.2020.00137. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32848600 Free PMC article. Review.
Cited by
-
Alzheimer's Disease Protein Targets: Comprehensive Review and Future Directions.Curr Pharm Des. 2025;31(17):1347-1369. doi: 10.2174/0113816128334916241006195142. Curr Pharm Des. 2025. PMID: 39428938 Review.
-
RHO to the DOCK for GDP disembarking: Structural insights into the DOCK GTPase nucleotide exchange factors.J Biol Chem. 2021 Jan-Jun;296:100521. doi: 10.1016/j.jbc.2021.100521. Epub 2021 Mar 5. J Biol Chem. 2021. PMID: 33684443 Free PMC article. Review.
-
Natural Product and Natural Product-Derived Gamma Secretase Modulators from Actaea Racemosa Extracts.Medicines (Basel). 2015 Jun 30;2(3):127-140. doi: 10.3390/medicines2030127. Medicines (Basel). 2015. PMID: 28930205 Free PMC article. Review.
-
Abeta, oxidative stress in Alzheimer disease: evidence based on proteomics studies.Biochim Biophys Acta. 2014 Aug;1842(8):1248-57. doi: 10.1016/j.bbadis.2013.09.015. Epub 2013 Oct 9. Biochim Biophys Acta. 2014. PMID: 24120836 Free PMC article. Review.
-
Association of CSF Aβ38 Levels With Risk of Alzheimer Disease-Related Decline.Neurology. 2022 Mar 1;98(9):e958-e967. doi: 10.1212/WNL.0000000000013228. Epub 2021 Dec 22. Neurology. 2022. PMID: 34937781 Free PMC article. Clinical Trial.
References
-
- Kakuda N, Funamoto S, Yagishita S, et al. Equimolar production of amyloid β-protein and amyloid precursor protein intracellular domain from β-carboxyl-terminal fragment by γ-secretase. Journal of Biological Chemistry. 2006;281(21):14776–14786. - PubMed
-
- Sato T, Dohmae N, Qi Y, et al. Potential link between amyloid β-protein 42 and C-terminal fragment γ 49-99 of β-amyloid precursor protein. Journal of Biological Chemistry. 2003;278(27):24294–20301. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

