Prostate cancer stem-like cells/cancer-initiating cells have an autocrine system of hepatocyte growth factor
- PMID: 23320511
- PMCID: PMC7657102
- DOI: 10.1111/cas.12104
Prostate cancer stem-like cells/cancer-initiating cells have an autocrine system of hepatocyte growth factor
Abstract
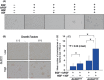
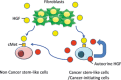
Prostate cancer cells include a small population of cancer stem-like cells (CSCs)/cancer-initiating cells (CICs) that have roles in initiation and progression of the cancer. Recently, we isolated prostate CSCs/CICs as aldehyde dehydrogenase 1-highh (ALDH1(high) ) cells using the ALDEFLUOR assay; however, the molecular mechanisms of prostate CSCs/CICs are still elusive. Prostate CSCs/CICs were isolated as ALDH1(high) cells using the ALDEFLUOR assay, and the gene expression profiles were analyzed using a cDNA microarray and RT-PCR. We found that prostate CSCs/CICs expressed higher levels of growth factors including hepatocyte growth factor (HGF). Hepatocyte growth factor protein expression was confirmed by enzyme linked immunosorbent assay and Western blotting. On the other hand, c-MET HGF receptor was expressed in both CSCs/CICs and non-CSCs/CICs at similar levels. Hepatocyte growth factor and the supernatant of myofibloblasts derived from the prostate augmented prostasphere formation in vitro, and prostasphere formation was inhibited by an anti-HGF antibody. Furthermore, c-MET gene knockdown by siRNA inhibited the prostasphere-forming ability in vitro and tumor-initiating ability in vivo. Taken together, the results indicate that HGF secreted by prostate CSCs/CICs and prostate myofibroblasts has a role in the maintenance of prostate CSCs/CICs in an autocrine and paracrine fashion.
© 2013 Japanese Cancer Association.
Figures




Similar articles
-
Small proline-rich protein-1B is overexpressed in human oral squamous cell cancer stem-like cells and is related to their growth through activation of MAP kinase signal.Biochem Biophys Res Commun. 2013 Sep 13;439(1):96-102. doi: 10.1016/j.bbrc.2013.08.021. Epub 2013 Aug 13. Biochem Biophys Res Commun. 2013. PMID: 23954638
-
Expression of hepatocyte growth factor in prostate cancer may indicate a biochemical recurrence after radical prostatectomy.Anticancer Res. 2015 Jan;35(1):413-8. Anticancer Res. 2015. PMID: 25550581
-
Fibroblasts induce expression of FGF4 in ovarian cancer stem-like cells/cancer-initiating cells and upregulate their tumor initiation capacity.Lab Invest. 2014 Dec;94(12):1355-69. doi: 10.1038/labinvest.2014.122. Epub 2014 Oct 20. Lab Invest. 2014. PMID: 25329002
-
C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell.Oral Oncol. 2014 Jul;50(7):633-9. doi: 10.1016/j.oraloncology.2014.04.004. Epub 2014 May 15. Oral Oncol. 2014. PMID: 24835851 Review.
-
Hepatocyte growth factor/MET in cancer progression and biomarker discovery.Cancer Sci. 2017 Mar;108(3):296-307. doi: 10.1111/cas.13156. Cancer Sci. 2017. PMID: 28064454 Free PMC article. Review.
Cited by
-
Roles of c-Met and RON kinases in tumor progression and their potential as therapeutic targets.Oncotarget. 2015 Feb 28;6(6):3507-18. doi: 10.18632/oncotarget.3420. Oncotarget. 2015. PMID: 25784650 Free PMC article. Review.
-
MET expression during prostate cancer progression.Oncotarget. 2016 May 24;7(21):31029-36. doi: 10.18632/oncotarget.8829. Oncotarget. 2016. PMID: 27105539 Free PMC article.
-
The Contributions of Prostate Cancer Stem Cells in Prostate Cancer Initiation and Metastasis.Cancers (Basel). 2019 Mar 27;11(4):434. doi: 10.3390/cancers11040434. Cancers (Basel). 2019. PMID: 30934773 Free PMC article. Review.
-
Inhibition of autocrine HGF maturation overcomes cetuximab resistance in colorectal cancer.Cell Mol Life Sci. 2024 Jan 12;81(1):28. doi: 10.1007/s00018-023-05071-5. Cell Mol Life Sci. 2024. PMID: 38212428 Free PMC article.
-
Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications.Medicines (Basel). 2019 Jul 30;6(3):82. doi: 10.3390/medicines6030082. Medicines (Basel). 2019. PMID: 31366128 Free PMC article. Review.
References
-
- Hirohashi Y, Torigoe T, Inoda S et al Immune response against tumor antigens expressed on human cancer stem‐like cells/tumor‐initiating cells. Immunotherapy 2010; 2: 201–11. - PubMed
-
- Nishida S, Hirohashi Y, Torigoe T et al Gene expression profiles of prostate cancer stem cells isolated by ALDH activity assay. J Urol 2012; 188: 294–9. - PubMed
-
- Inoda S, Hirohashi Y, Torigoe T et al Cep55/c10orf3, a tumor antigen derived from a centrosome residing protein in breast carcinoma. J Immunother 2009; 32: 474–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

