Structural factors controlling the spin-spin exchange coupling: EPR spectroscopic studies of highly asymmetric trityl-nitroxide biradicals
- PMID: 23320522
- PMCID: PMC4073598
- DOI: 10.1021/ja311571v
Structural factors controlling the spin-spin exchange coupling: EPR spectroscopic studies of highly asymmetric trityl-nitroxide biradicals
Abstract
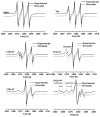
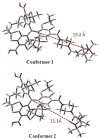
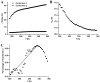
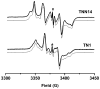
Highly asymmetric exchange-coupled biradicals, e.g., the trityl-nitroxides (TNs), possess particular magnetic properties that have opened new possibilities for their application in biophysical, physicochemical, and biological studies. In the present work, we investigated the effect of the linker length on the spin-spin coupling interaction (J) in TN biradicals using the newly synthesized biradicals CT02-GT, CT02-AT, CT02-VT, and CT02-PPT as well as the previously reported biradicals TNN14 and TN1. The results show that the magnitude of J can be easily tuned from ~4 G (conformer 1 in CT02-PPT) to >1200 G (in TNN14) by varying the linker separating the two radical moieties and changing the temperature. Computer simulations of EPR spectra were carried out to estimate J values of the TN biradicals directly. In addition to the spin-spin coupling interaction of TN biradicals, their g, hyperfine-splitting, and zero-field-splitting interactions were explored at low temperature (220 K). Our present study clearly shows that varying the spin-spin interaction as a function of linker distance and temperature provides an effective strategy for the development of new TN biradicals that can find wide applications in relevant fields.
Figures



References
-
- Song CS, Hu KN, Joo CG, Swager TM, Griffin RG. J Am Chem Soc. 2006;128:11385–11390. - PubMed
-
- Zagdoun A, Casano G, Ouari O, Lapadula G, Rossini AJ, Lelli M, Baffert M, Gajan D, Veyre L, Maas WE, Rosay M, Weber RT, Thieuleux C, Coperet C, Lesage A, Tordo P, Emsley L. J Am Chem Soc. 2012;134:2284–2291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

