Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi
- PMID: 23320757
- PMCID: PMC3556326
- DOI: 10.1186/1472-6750-13-3
Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi
Abstract
Background: Halorubrum lacusprofundi is a cold-adapted halophilic archaeon isolated from Deep Lake, a perennially cold and hypersaline lake in Antarctica. Its genome sequencing project was recently completed, providing access to many genes predicted to encode polyextremophilic enzymes active in both extremely high salinity and cold temperatures.
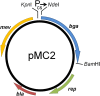
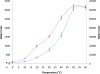
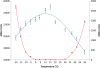
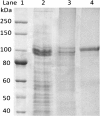
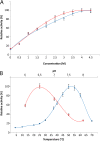
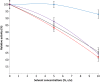
Results: Analysis of the genome sequence of H. lacusprofundi showed a gene cluster for carbohydrate utilization containing a glycoside hydrolase family 42 β-galactosidase gene, named bga. In order to study the biochemical properties of the β-galactosidase enzyme, the bga gene was PCR amplified, cloned, and expressed in the genetically tractable haloarchaeon Halobacterium sp. NRC-1 under the control of a cold shock protein (cspD2) gene promoter. The recombinant β-galactosidase protein was produced at 20-fold higher levels compared to H. lacusprofundi, purified using gel filtration and hydrophobic interaction chromatography, and identified by SDS-PAGE, LC-MS/MS, and ONPG hydrolysis activity. The purified enzyme was found to be active over a wide temperature range (-5 to 60°C) with an optimum of 50°C, and 10% of its maximum activity at 4°C. The enzyme also exhibited extremely halophilic character, with maximal activity in either 4 M NaCl or KCl. The polyextremophilic β-galactosidase was also stable and active in 10-20% alcohol-aqueous solutions, containing methanol, ethanol, n-butanol, or isoamyl alcohol.
Conclusion: The H. lacusprofundi β-galactosidase is a polyextremophilic enzyme active in high salt concentrations and low and high temperature. The enzyme is also active in aqueous-organic mixed solvents, with potential applications in synthetic chemistry. H. lacuprofundi proteins represent a significant biotechnology resource and for developing insights into enzyme catalysis under water limiting conditions. This study provides a system for better understanding how H. lacusprofundi is successful in a perennially cold, hypersaline environment, with relevance to astrobiology.
Figures

Similar articles
-
Key amino acid residues conferring enhanced enzyme activity at cold temperatures in an Antarctic polyextremophilic β-galactosidase.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):12530-12535. doi: 10.1073/pnas.1711542114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109294 Free PMC article.
-
Amino acid substitutions in cold-adapted proteins from Halorubrum lacusprofundi, an extremely halophilic microbe from antarctica.PLoS One. 2013;8(3):e58587. doi: 10.1371/journal.pone.0058587. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23536799 Free PMC article.
-
Understanding High-Salt and Cold Adaptation of a Polyextremophilic Enzyme.Microorganisms. 2020 Oct 16;8(10):1594. doi: 10.3390/microorganisms8101594. Microorganisms. 2020. PMID: 33081237 Free PMC article.
-
Extremophilic models for astrobiology: haloarchaeal survival strategies and pigments for remote sensing.Extremophiles. 2020 Jan;24(1):31-41. doi: 10.1007/s00792-019-01126-3. Epub 2019 Aug 28. Extremophiles. 2020. PMID: 31463573 Free PMC article. Review.
-
A Review on Psychrophilic β-D-Galactosidases and Their Potential Applications.Appl Biochem Biotechnol. 2023 Apr;195(4):2743-2766. doi: 10.1007/s12010-022-04215-w. Epub 2022 Nov 23. Appl Biochem Biotechnol. 2023. PMID: 36422804 Review.
Cited by
-
Marine extremophiles: a source of hydrolases for biotechnological applications.Mar Drugs. 2015 Apr 3;13(4):1925-65. doi: 10.3390/md13041925. Mar Drugs. 2015. PMID: 25854643 Free PMC article. Review.
-
Activation of LacZ gene in Escherichia coli DH5α via α-complementation mechanism for β-galactosidase production and its biochemical characterizations.J Genet Eng Biotechnol. 2020 Dec 2;18(1):80. doi: 10.1186/s43141-020-00096-w. J Genet Eng Biotechnol. 2020. PMID: 33263861 Free PMC article.
-
Identification and characterization of thermophilic amylase producing bacterial isolates from the brick kiln soil.Saudi J Biol Sci. 2021 Jan;28(1):970-979. doi: 10.1016/j.sjbs.2020.11.017. Epub 2020 Nov 11. Saudi J Biol Sci. 2021. PMID: 33424389 Free PMC article.
-
Industrial Biotechnology Based on Enzymes From Extreme Environments.Front Bioeng Biotechnol. 2022 Apr 5;10:870083. doi: 10.3389/fbioe.2022.870083. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35480975 Free PMC article. Review.
-
Crystal Structure and Active Site Engineering of a Halophilic γ-Carbonic Anhydrase.Front Microbiol. 2020 Apr 28;11:742. doi: 10.3389/fmicb.2020.00742. eCollection 2020. Front Microbiol. 2020. PMID: 32411108 Free PMC article.
References
-
- Franzmann PD, Stackebrandt E, Sanderson K, Volkman JK, Cameron DE, Stevenson PL, McMeekin TA, Burton HR. Halobacterium lacusprofundi, sp. nov, a halophilic bacterium isolated from Deep Lake, Antarctica. Syst Appl Microbiol. 1988;11:20–27. doi: 10.1016/S0723-2020(88)80044-4. - DOI
-
- Reid IN, Sparks WB, Lubow S, McGrath M, Livio M, Valenti J, Sowers KR, Shukla HD, MacAuley S, Miller T, Suvanasuthi R, Belas R, Colman A, Robb FT, DasSarma P, Müller JA, Coker JA, Cavicchioli R, Chen F, DasSarma S. Terrestrial models for extraterrestrial life: methanogens and halophiles at Martian temperatures. Intl J Astrobiol. 2006;5:89–97. doi: 10.1017/S1473550406002916. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

