TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier
- PMID: 23320797
- PMCID: PMC3570290
- DOI: 10.1186/1742-2094-10-9
TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier
Abstract
Background: The TNF ligand family member TWEAK exists as membrane and soluble forms and is involved in the regulation of various human inflammatory pathologies, through binding to its main receptor, Fn14. We have shown that the soluble form of TWEAK has a pro-neuroinflammatory effect in an animal model of multiple sclerosis and we further demonstrated that blocking TWEAK activity during the recruitment phase of immune cells across the blood brain barrier (BBB) was protective in this model. It is now well established that endothelial cells in the periphery and astrocytes in the central nervous system (CNS) are targets of TWEAK. Moreover, it has been shown by others that, when injected into mice brains, TWEAK disrupts the architecture of the BBB and induces expression of matrix metalloproteinase-9 (MMP-9) in the brain. Nevertheless, the mechanisms involved in such conditions are complex and remain to be explored, especially because there is a lack of data concerning the TWEAK/Fn14 pathway in microvascular cerebral endothelial cells.
Methods: In this study, we used human cerebral microvascular endothelial cell (HCMEC) cultures as an in vitro model of the BBB to study the effects of soluble TWEAK on the properties and the integrity of the BBB model.
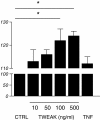
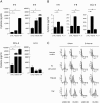
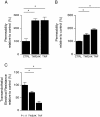
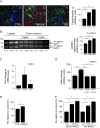
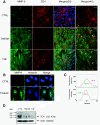
Results: We showed that soluble TWEAK induces an inflammatory profile on HCMECs, especially by promoting secretion of cytokines, by modulating production and activation of MMP-9, and by expression of cell adhesion molecules. We also demonstrated that these effects of TWEAK are associated with increased permeability of the HCMEC monolayer in the in vitro BBB model.
Conclusions: Taken together, the data suggest a role for soluble TWEAK in BBB inflammation and in the promotion of BBB interactions with immune cells. These results support the contention that the TWEAK/Fn14 pathway could contribute at least to the endothelial steps of neuroinflammation.
Figures
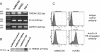
References
-
- Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179:7949–7958. - PubMed
-
- van Kuijk AWR, Wijbrandts CA, Vinkenoog M, Zheng TS, Reedquist KA, Tak PP. TWEAK and its receptor Fn14 in the synovium of patients with rheumatoid arthritis compared to psoriatic arthritis and its response to tumour necrosis factor blockade. Ann Rheum Dis. 2010;69:301–304. doi: 10.1136/ard.2008.090548. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

