Arabidopsis TWISTED DWARF1 functionally interacts with auxin exporter ABCB1 on the root plasma membrane
- PMID: 23321285
- PMCID: PMC3584535
- DOI: 10.1105/tpc.112.105999
Arabidopsis TWISTED DWARF1 functionally interacts with auxin exporter ABCB1 on the root plasma membrane
Abstract
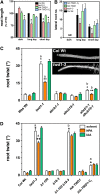
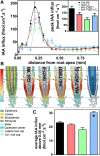
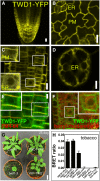
Plant architecture is influenced by the polar, cell-to-cell transport of auxin that is primarily provided and regulated by plasma membrane efflux catalysts of the PIN-FORMED and B family of ABC transporter (ABCB) classes. The latter were shown to require the functionality of the FK506 binding protein42 TWISTED DWARF1 (TWD1), although underlying mechanisms are unclear. By genetic manipulation of TWD1 expression, we show here that TWD1 affects shootward root auxin reflux and, thus, downstream developmental traits, such as epidermal twisting and gravitropism of the root. Using immunological assays, we demonstrate a predominant lateral, mainly outward-facing, plasma membrane location for TWD1 in the root epidermis characterized by the lateral marker ABC transporter G36/PLEIOTROPIC DRUG-RESISTANCE8/PENETRATION3. At these epidermal plasma membrane domains, TWD1 colocalizes with nonpolar ABCB1. In planta bioluminescence resonance energy transfer analysis was used to verify specific ABC transporter B1 (ABCB1)-TWD1 interaction. Our data support a model in which TWD1 promotes lateral ABCB-mediated auxin efflux via protein-protein interaction at the plasma membrane, minimizing reflux from the root apoplast into the cytoplasm.
Figures




Similar articles
-
Expression of TWISTED DWARF1 lacking its in-plane membrane anchor leads to increased cell elongation and hypermorphic growth.Plant J. 2014 Jan;77(1):108-18. doi: 10.1111/tpj.12369. Epub 2013 Dec 9. Plant J. 2014. PMID: 24313847
-
The ER-localized TWD1 immunophilin is necessary for localization of multidrug resistance-like proteins required for polar auxin transport in Arabidopsis roots.Plant Cell. 2010 Oct;22(10):3295-304. doi: 10.1105/tpc.110.078360. Epub 2010 Oct 22. Plant Cell. 2010. PMID: 20971896 Free PMC article.
-
Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins.J Biol Chem. 2008 Aug 1;283(31):21817-26. doi: 10.1074/jbc.M709655200. Epub 2008 May 22. J Biol Chem. 2008. PMID: 18499676
-
A Critical View on ABC Transporters and Their Interacting Partners in Auxin Transport.Plant Cell Physiol. 2017 Oct 1;58(10):1601-1614. doi: 10.1093/pcp/pcx104. Plant Cell Physiol. 2017. PMID: 29016918 Review.
-
Auxin transport and gravitational research: perspectives.Protoplasma. 2006 Dec;229(2-4):175-81. doi: 10.1007/s00709-006-0216-9. Epub 2006 Dec 16. Protoplasma. 2006. PMID: 17180499 Review.
Cited by
-
A transportome-scale amiRNA-based screen identifies redundant roles of Arabidopsis ABCB6 and ABCB20 in auxin transport.Nat Commun. 2018 Oct 11;9(1):4204. doi: 10.1038/s41467-018-06410-y. Nat Commun. 2018. PMID: 30310073 Free PMC article.
-
Integrative omics approaches revealed a crosstalk among phytohormones during tuberous root development in cassava.Plant Mol Biol. 2022 Jun;109(3):249-269. doi: 10.1007/s11103-020-01033-8. Epub 2020 Aug 5. Plant Mol Biol. 2022. PMID: 32757126
-
TWISTED DWARF1 Mediates the Action of Auxin Transport Inhibitors on Actin Cytoskeleton Dynamics.Plant Cell. 2016 Apr;28(4):930-48. doi: 10.1105/tpc.15.00726. Epub 2016 Apr 6. Plant Cell. 2016. PMID: 27053424 Free PMC article.
-
Genome-wide analyses of genes encoding FK506-binding proteins reveal their involvement in abiotic stress responses in apple.BMC Genomics. 2018 Sep 25;19(1):707. doi: 10.1186/s12864-018-5097-8. BMC Genomics. 2018. PMID: 30253753 Free PMC article.
-
Loss of Multiple ABCB Auxin Transporters Recapitulates the Major twisted dwarf 1 Phenotypes in Arabidopsis thaliana.Front Plant Sci. 2022 Apr 21;13:840260. doi: 10.3389/fpls.2022.840260. eCollection 2022. Front Plant Sci. 2022. PMID: 35528937 Free PMC article.
References
-
- Abas L., Benjamins R., Malenica N., Paciorek T., Wiśniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8: 249–256 Erratum. Nat. Cell Biol. 8: 424. - PubMed
-
- Bailly A., Sovero V., Vincenzetti V., Santelia D., Bartnik D., Koenig B.W., Mancuso S., Martinoia E., Geisler M. (2008). Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J. Biol. Chem. 283: 21817–21826 - PubMed
-
- Bouchard R., Bailly A., Blakeslee J.J., Oehring S.C., Vincenzetti V., Lee O.R., Paponov I., Palme K., Mancuso S., Murphy A.S., Schulz B., Geisler M. (2006). Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J. Biol. Chem. 281: 30603–30612 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

