Informed consent for whole-genome sequencing studies in the clinical setting. Proposed recommendations on essential content and process
- PMID: 23321621
- PMCID: PMC3778336
- DOI: 10.1038/ejhg.2012.297
Informed consent for whole-genome sequencing studies in the clinical setting. Proposed recommendations on essential content and process
Abstract
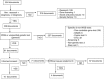
The development of new massive sequencing techniques has now made it possible to significantly reduce the time and costs of whole-genome sequencing (WGS). Although WGS will soon become a routine testing tool, new ethical issues have surfaced. In light of these concerns, a systematic review of papers published by expert authors on IC or specific ethical issues related to IC for WGS analysis in the clinical setting has been conducted using the Pubmed, Embase and Cochrane Library databases. Additionally, a search was conducted for international ethical guidelines for genetic studies published by scientific societies and ethical boards. Based on these documents, a minimum set of information to be provided to patients in the IC form was determined. Fourteen and seven documents from the database search and from scientific societies, respectively, were selected. A very high level of consistency between them was found regarding the recommended IC form content. Pre-test counselling and general information common to all genetic tests should be included in the IC form for WGS for diagnostic purposes, but additional information addressing specific issues on WGS are proposed, such as a plan for the ethical, clinically oriented return of incidental findings. Moreover, storage of additional information for future use should also be agreed upon with the patient in advance. Recommendations for WGS studies in the clinical setting concerning both the elements of information and the process of obtaining the IC as well as how to handle the results obtained are proposed.
Figures
Comment in
-
Reply to Townsend et al.Eur J Hum Genet. 2014 Jan;22(1):7. doi: 10.1038/ejhg.2013.95. Epub 2013 May 15. Eur J Hum Genet. 2014. PMID: 23674173 Free PMC article. No abstract available.
-
Autonomy and the patient's right 'not to know' in clinical whole-genomic sequencing.Eur J Hum Genet. 2014 Jan;22(1):6. doi: 10.1038/ejhg.2013.94. Epub 2013 May 15. Eur J Hum Genet. 2014. PMID: 23674174 Free PMC article. No abstract available.
References
-
- Human Genome Project Information , http://www.ornl.gov/sci/techresources/Human_Genome/home.shtml (Accessed 20 June 2012).
-
- National Human Genome Research Institute , http://www.genome.gov/11006943 (Accessed 20 June 2012).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

