Haloperidol and olanzapine mediate metabolic abnormalities through different molecular pathways
- PMID: 23321805
- PMCID: PMC3566719
- DOI: 10.1038/tp.2012.138
Haloperidol and olanzapine mediate metabolic abnormalities through different molecular pathways
Abstract
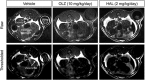
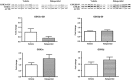
The pathogenesis of antipsychotic-induced disturbances of glucose homeostasis is still unclear. Increased visceral adiposity has been suggested to be a possible mediating mechanism. The aim of this study was to investigate, in an animal model, the differential effects of olanzapine and haloperidol on visceral fat deposition (using magnetic resonance imaging(MRI)) and on critical nodes of the insulin signaling pathway (liver-protein levels of IRS2 (insulin receptor substrate 2), GSK3α (glycogen synthase kinase-3α), GSK3β, GSK3α-Ser21, GSK3β-Ser9). To this end, we studied male Sprague-Dawley rats treated with vehicle (n=8), haloperidol (2 mg kg(-1) per day, n=8), or olanzapine (10 mg kg(-1)per day, n=8), using osmotic minipumps, for 8 weeks. The haloperidol group showed a higher percentage of visceral fat than both the olanzapine group and the vehicle group, whereas there was no difference between the olanzapine and the vehicle group. In terms of insulin signaling pathway, the olanzapine group showed significantly reduced IRS2 levels, reduced phosphorylation of GSK3α and increased phosphorylation of GSK3β, whereas there was no difference between the haloperidol and the vehicle group. Our data suggest that different molecular pathways mediate the disturbances of glucose homeostasis induced by haloperidol and olanzapine with a direct effect of olanzapine on the insulin molecular pathway, possibly partly explaining the stronger propensity of olanzapine for adverse effects on glucose regulation when compared with haloperidol in clinical settings.
Figures


References
-
- De Hert M, Cohen D, Bobes J, Cetkovich-Bakmas M, Leucht S, Ndetei DM, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10:138–151. - PMC - PubMed
-
- Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–217. - PubMed
-
- Heiskanen T, Niskanen L, Lyytikainen R, Saarinen PI, Hintikka J. Metabolic syndrome in patients with schizophrenia. J Clin Psychiatry. 2003;64:575–579. - PubMed
-
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19 (Suppl 1:1–93. - PubMed
-
- Smith M, Hopkins D, Peveler RC, Holt RI, Woodward M, Ismail K. First- v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2008;192:406–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

