RNAi-mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis
- PMID: 23321808
- PMCID: PMC3566716
- DOI: 10.1038/tp.2012.135
RNAi-mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis
Abstract
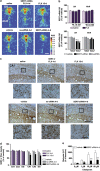
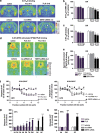
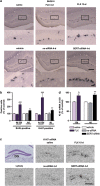
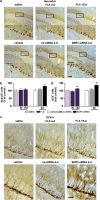
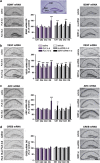
Current antidepressants, which inhibit the serotonin transporter (SERT), display limited efficacy and slow onset of action. Here, we show that partial reduction of SERT expression by small interference RNA (SERT-siRNA) decreased immobility in the tail suspension test, displaying an antidepressant potential. Moreover, short-term SERT-siRNA treatment modified mouse brain variables considered to be key markers of antidepressant action: reduced expression and function of 5-HT(1A)-autoreceptors, elevated extracellular serotonin in forebrain and increased neurogenesis and expression of plasticity-related genes (BDNF, VEGF, Arc) in hippocampus. Remarkably, these effects occurred much earlier and were of greater magnitude than those evoked by long-term fluoxetine treatment. These findings highlight the critical role of SERT in serotonergic function and show that the reduction of SERT expression regulates serotonergic neurotransmission more potently than pharmacological blockade of SERT. The use of siRNA-targeting genes in serotonin neurons (SERT, 5-HT(1A)-autoreceptor) may be a novel therapeutic strategy to develop fast-acting antidepressants.
Figures
Similar articles
-
Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects.Mol Psychiatry. 2012 Jun;17(6):612-23. doi: 10.1038/mp.2011.92. Epub 2011 Aug 2. Mol Psychiatry. 2012. PMID: 21808255
-
Chronic fluoxetine rescues changes in plasma membrane density of 5-HT1A autoreceptors and serotonin transporters in the olfactory bulbectomy rodent model of depression.Neuroscience. 2017 Jul 25;356:78-88. doi: 10.1016/j.neuroscience.2017.05.021. Epub 2017 May 19. Neuroscience. 2017. PMID: 28528967
-
Acute 5-HT₁A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions.Psychopharmacology (Berl). 2013 Jan;225(1):61-74. doi: 10.1007/s00213-012-2795-9. Epub 2012 Jul 21. Psychopharmacology (Berl). 2013. PMID: 22820867
-
Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT.Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2416-25. doi: 10.1098/rstb.2011.0361. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22826342 Free PMC article. Review.
-
Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review.
Cited by
-
Dynamic changes of serotonin transporter expression in the prefrontal cortex evoked by aggressive social interactions.Neurobiol Stress. 2025 Mar 26;36:100722. doi: 10.1016/j.ynstr.2025.100722. eCollection 2025 May. Neurobiol Stress. 2025. PMID: 40230625 Free PMC article.
-
β-Catenin Role in the Vulnerability/Resilience to Stress-Related Disorders Is Associated to Changes in the Serotonergic System.Mol Neurobiol. 2020 Mar;57(3):1704-1715. doi: 10.1007/s12035-019-01841-0. Epub 2019 Dec 10. Mol Neurobiol. 2020. PMID: 31823197
-
Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration.Mol Psychiatry. 2016 Mar;21(3):328-38. doi: 10.1038/mp.2015.80. Epub 2015 Jun 23. Mol Psychiatry. 2016. PMID: 26100539 Free PMC article.
-
Actions of Brain-Derived Neurotrophic Factor and Glucocorticoid Stress in Neurogenesis.Int J Mol Sci. 2017 Nov 2;18(11):2312. doi: 10.3390/ijms18112312. Int J Mol Sci. 2017. PMID: 29099059 Free PMC article. Review.
-
A critical evaluation of the activity-regulated cytoskeleton-associated protein (Arc/Arg3.1)'s putative role in regulating dendritic plasticity, cognitive processes, and mood in animal models of depression.Front Neurosci. 2015 Aug 10;9:279. doi: 10.3389/fnins.2015.00279. eCollection 2015. Front Neurosci. 2015. PMID: 26321903 Free PMC article. Review.
References
-
- Smith K. Trillion-dollar brain drain. Nature. 2011;478:15. - PubMed
-
- Wong ML, Licinio J. Research and treatment approaches to depression. Nature Rev Neurosci. 2001;2:343–351. - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

