REG Iα is a biomarker for predicting response to chemotherapy with S-1 plus cisplatin in patients with unresectable stage IV gastric cancer
- PMID: 23322208
- PMCID: PMC3566803
- DOI: 10.1038/bjc.2012.572
REG Iα is a biomarker for predicting response to chemotherapy with S-1 plus cisplatin in patients with unresectable stage IV gastric cancer
Abstract
Background: The regenerating gene Iα (REG Iα) is involved in gastric carcinogenesis as an antiapoptotic factor. Therefore, we investigated whether REG Iα confers resistance to chemotherapeutic drugs in gastric cancer (GC) cells and whether REG Iα expression is useful for predicting the response to chemotherapy and outcome in patients with GC.
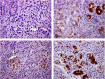
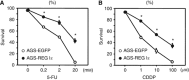
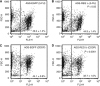
Methods: A total of 70 patients with unresectable stage IV GC received first-line chemotherapy with S-1 and cisplatin (S-1/CDDP). The expression of REG Iα was evaluated immunohistochemically using biopsy samples obtained before chemotherapy, and its relationship to clinicopathological parameters was analysed statistically. The effects of REG Iα gene induction on resistance to 5-FU or CDDP treatment were examined by cell survival assay and flow cytometry.
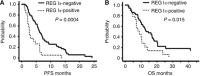
Results: Of the 70 patients with unresectable stage IV GC, 19 (27%) were positive for REG Iα expression. The expression of REG Iα was independently predictive of poorer progression-free and overall survival in such patients (hazard ratio (HR) 2.46; P=0.002 and HR 1.89; P=0.037, respectively). The gene induction of REG Iα conferred resistance to cell death induced by 5-FU or CDDP in GC cells.
Conclusion: In patients with stage IV GC, REG Iα, which confers resistance to chemotherapeutic drugs in GC cells, is a potential biomarker for predicting resistance to S-1/CDDP treatment.
Figures
References
-
- Boku N. Chemotherapy for metastatic gastric cancer in Japan. Int J Oncol. 2008;13:483–487. - PubMed
-
- Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer-pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. - PubMed
-
- Choi IS, Lee HS, Lee KW, Kim H, Kim KH, Kim YJ, Kim JH, Kim WH, Lee JS. Biomarker analysis in patients with advanced gastric cancer treated with S-1 plus cisplatin chemotherapy: orotate phosphoribosyltransferase expression is associated with treatment outcomes. Med Oncol. 2011;28:991–998. - PubMed
-
- Dhar DK, Udagawa J, Ishihara S, Otani H, Kinoshita Y, Takasawa S, Okamoto H, Kubota H, Fujii T, Tachibana M, Nagasue N. Expression of regenerating gene I in gastric cancer adenocarcinomas. Cancer. 2004;100:1130–1136. - PubMed
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

