FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration
- PMID: 23322300
- PMCID: PMC3566310
- DOI: 10.1101/gad.204776.112
FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration
Abstract
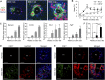
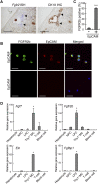
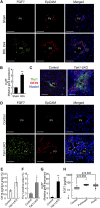
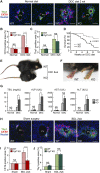
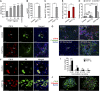
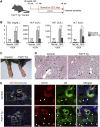
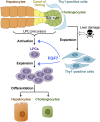
The liver is a unique organ with a remarkably high potential to regenerate upon injuries. In severely damaged livers where hepatocyte proliferation is impaired, facultative liver progenitor cells (LPCs) proliferate and are assumed to contribute to regeneration. An expansion of LPCs is often observed in patients with various types of liver diseases. However, the underlying mechanism of LPC activation still remains largely unknown. Here we show that a member of the fibroblast growth factor (FGF) family, FGF7, is a critical regulator of LPCs. Its expression was induced concomitantly with LPC response in the liver of mouse models as well as in the serum of patients with acute liver failure. Fgf7-deficient mice exhibited markedly depressed LPC expansion and higher mortality upon toxin-induced hepatic injury. Transgenic expression of FGF7 in vivo led to the induction of cells with characteristics of LPCs and ameliorated hepatic dysfunction. We revealed that Thy1(+) mesenchymal cells produced FGF7 and appeared in close proximity to LPCs, implicating a role for those cells as the functional LPC niche in the regenerating liver. These findings provide new insights into the cellular and molecular basis for LPC regulation and identify FGF7 as a potential therapeutic target for liver diseases.
Figures
Similar articles
-
Hdac1 Regulates Differentiation of Bipotent Liver Progenitor Cells During Regeneration via Sox9b and Cdk8.Gastroenterology. 2019 Jan;156(1):187-202.e14. doi: 10.1053/j.gastro.2018.09.039. Epub 2018 Sep 26. Gastroenterology. 2019. PMID: 30267710 Free PMC article.
-
Transplantation of Thy1+ Cells Accelerates Liver Regeneration by Enhancing the Growth of Small Hepatocyte-Like Progenitor Cells via IL17RB Signaling.Stem Cells. 2017 Apr;35(4):920-931. doi: 10.1002/stem.2548. Epub 2017 Jan 19. Stem Cells. 2017. PMID: 27925343
-
Expression and function of fibroblast growth factor (FGF) 7 during liver regeneration.Cell Physiol Biochem. 2011;27(6):641-52. doi: 10.1159/000330073. Epub 2011 Jun 17. Cell Physiol Biochem. 2011. PMID: 21691082
-
Liver progenitor cell-driven liver regeneration.Exp Mol Med. 2020 Aug;52(8):1230-1238. doi: 10.1038/s12276-020-0483-0. Epub 2020 Aug 14. Exp Mol Med. 2020. PMID: 32796957 Free PMC article. Review.
-
The progenitor cell dilemma: Cellular and functional heterogeneity in assistance or escalation of liver injury.J Hepatol. 2017 Mar;66(3):619-630. doi: 10.1016/j.jhep.2016.10.033. Epub 2016 Nov 5. J Hepatol. 2017. PMID: 27826058 Review.
Cited by
-
Liver regeneration and fibrosis after inflammation.Inflamm Regen. 2016 Oct 18;36:19. doi: 10.1186/s41232-016-0025-2. eCollection 2016. Inflamm Regen. 2016. PMID: 29259692 Free PMC article. Review.
-
Roles of FGFs As Paracrine or Endocrine Signals in Liver Development, Health, and Disease.Front Cell Dev Biol. 2016 Apr 13;4:30. doi: 10.3389/fcell.2016.00030. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27148532 Free PMC article. Review.
-
Fibroblast growth factor signaling in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Paving the way to hepatocellular carcinoma.World J Gastroenterol. 2020 Jan 21;26(3):279-290. doi: 10.3748/wjg.v26.i3.279. World J Gastroenterol. 2020. PMID: 31988589 Free PMC article. Review.
-
Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling.Elife. 2016 Jul 19;5:e15034. doi: 10.7554/eLife.15034. Elife. 2016. PMID: 27431614 Free PMC article.
-
Fibroblast growth factor signaling regulates the expansion of A6-expressing hepatocytes in association with AKT-dependent β-catenin activation.J Hepatol. 2014 May;60(5):1002-9. doi: 10.1016/j.jhep.2013.12.017. Epub 2013 Dec 21. J Hepatol. 2014. PMID: 24365171 Free PMC article.
References
-
- Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, Yeoh GC 2001. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology 34: 519–522 - PubMed
-
- Alison MR, Golding MH, Sarraf CE 1996. Pluripotential liver stem cells: Facultative stem cells located in the biliary tree. Cell Prolif 29: 373–402 - PubMed
-
- Beaven AW, Shea TC 2007. The effect of palifermin on chemotherapyand radiation therapy-induced mucositis: A review of the current literature. Support Cancer Ther 4: 188–197 - PubMed
-
- Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S 2005. The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: Implications for epithelial repair. Oncogene 24: 5269–5277 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
