Compensatory regulation of the Snai1 and Snai2 genes during chondrogenesis
- PMID: 23322385
- PMCID: PMC3663919
- DOI: 10.1002/jbmr.1871
Compensatory regulation of the Snai1 and Snai2 genes during chondrogenesis
Abstract
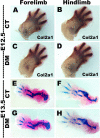
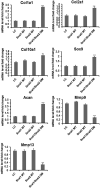
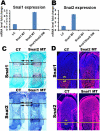
Endochondral bone formation is a multistep process during which a cartilage primordium is replaced by mineralized bone. Several genes involved in cartilage and bone development have been identified as target genes for the Snail family of zinc finger transcriptional repressors, and a gain-of-function study has demonstrated that upregulation of Snai1 activity in mouse long bones caused a reduction in bone length. However, no in vivo loss-of-function studies have been performed to establish whether Snail family genes have an essential, physiological role during normal bone development. We demonstrate here that the Snai1 and Snai2 genes function redundantly during embryonic long bone development in mice. Deletion of the Snai2 gene, or limb bud-specific conditional deletion of the Snai1 gene, did not result in obvious defects in the skeleton. However, limb bud-specific Snai1 deletion on a Snai2 null genetic background resulted in substantial defects in the long bones of the limbs. Long bones of the Snai1/Snai2 double mutants exhibited defects in chondrocyte morphology and organization, inhibited trabecular bone formation, and delayed ossification. Chondrocyte proliferation was markedly reduced, and transcript levels of genes encoding cell cycle regulators, such as p21(Waf1/Cip1) , were strikingly upregulated in the Snai1/Snai2 double mutants, suggesting that during chondrogenesis Snail family proteins act to control cell proliferation by mediating expression of cell-cycle regulators. Snai2 transcript levels were increased in Snai1 mutant femurs, whereas Snai1 transcript levels were increased in Snai2 mutant femurs. In addition, in the mutant femurs the Snai1 and Snai2 genes compensated for each other's loss not only quantitatively, but also by expanding their expression into the other genes' normal expression domains. These results demonstrate that the Snai1 and Snai2 genes transcriptionally compensate temporally, spatially, and quantitatively for each other's loss, and demonstrate an essential role for Snail family genes during chondrogenesis in mice.
Copyright © 2013 American Society for Bone and Mineral Research.
Figures




References
-
- Hartmann C. Transcriptional networks controlling skeletal development. Curr Opin Genet Dev. 2009;19:437–43. - PubMed
-
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–48. - PubMed
-
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

