Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system
- PMID: 23322571
- PMCID: PMC3576052
- DOI: 10.1167/iovs.12-11172
Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system
Abstract
Purpose: To test the feasibility of covalent loading of daunorubicin into oxidized porous silicon (OPS) and to evaluate the ocular properties of sustained delivery of daunorubicin in this system.
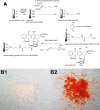
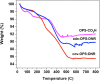
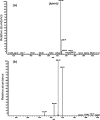
Methods: Porous silicon was heat oxidized and chemically functionalized so that the functional linker on the surface was covalently bonded with daunorubicin. The drug loading rate was determined by thermogravimetric analysis. Release of daunorubicin was confirmed in PBS and excised rabbit vitreous by mass spectrometry. Daunorubicin-loaded OPS particles (3 mg) were intravitreally injected into six rabbits, and ocular properties were evaluated through ophthalmic examinations and histology during a 3-month study. The same OPS was loaded with daunorubicin using physical adsorption and was evaluated similarly as a control for the covalent loading.
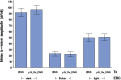
Results: In the case of covalent loading, 67 ± 10 μg daunorubicin was loaded into each milligram of the particles while 27 ± 10 μg/mg particles were loaded by physical adsorption. Rapid release of daunorubicin was observed in both PBS and excised vitreous (~75% and ~18%) from the physical adsorption loading, while less than 1% was released from the covalently loaded particles. Following intravitreal injection, the covalently loaded particles demonstrated a sustained degradation of OPS with drug release for 3 months without evidence of toxicity; physical adsorption loading revealed a complete release within 2 weeks and localized retinal toxicity due to high daunorubicin concentration.
Conclusions: OPS with covalently loaded daunorubicin demonstrated sustained intravitreal drug release without ocular toxicity, which may be useful to inhibit unwanted intraocular proliferation.
Conflict of interest statement
Disclosure:
Figures








References
-
- Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998; 43: 3–18 - PubMed
-
- Turgut B, Uyar F, Ustundag B, Celiker U, Akpolat N, Demir T. The impact of tacrolimus on growth factors in experimental proliferative vitreoretinopathy. Retina. 2012; 32: 232–241 - PubMed
-
- Wiedemann P, Sorgente N, Bekhor C, Patterson R, Tran T, Ryan SJ. Daunomycin in the treatment of experimental proliferative vitreoretinopathy. Effective doses in vitro and in vivo. Invest Ophthalmol Vis Sci. 1985; 26: 719–725 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

