Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice
- PMID: 23322710
- PMCID: PMC3638069
- DOI: 10.1002/hep.26267
Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice
Abstract
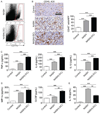
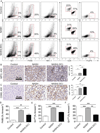
Nonalcoholic steatohepatitis (NASH) is the most common etiology of chronic liver dysfunction in the United States and can progress to cirrhosis and liver failure. Inflammatory insult resulting from fatty infiltration of the liver is central to disease pathogenesis. Dendritic cells (DCs) are antigen-presenting cells with an emerging role in hepatic inflammation. We postulated that DCs are important in the progression of NASH. We found that intrahepatic DCs expand and mature in NASH liver and assume an activated immune phenotype. However, rather than mitigating the severity of NASH, DC depletion markedly exacerbated intrahepatic fibroinflammation. Our mechanistic studies support a regulatory role for DCs in NASH by limiting sterile inflammation through their role in the clearance of apoptotic cells and necrotic debris. We found that DCs limit CD8(+) T-cell expansion and restrict Toll-like receptor expression and cytokine production in innate immune effector cells in NASH, including Kupffer cells, neutrophils, and inflammatory monocytes. Consistent with their regulatory role in NASH, during the recovery phase of disease, ablation of DC populations results in delayed resolution of intrahepatic inflammation and fibroplasia.
Conclusion: Our findings support a role for DCs in modulating NASH. Targeting DC functional properties may hold promise for therapeutic intervention in NASH.
Copyright © 2013 American Association for the Study of Liver Diseases.
Figures





Comment in
-
From NAFLD to NASH to fibrosis to HCC: role of dendritic cell populations in the liver.Hepatology. 2013 Aug;58(2):494-6. doi: 10.1002/hep.26405. Epub 2013 Jun 25. Hepatology. 2013. PMID: 23519833 No abstract available.
-
Dendritic cells in NASH: friend or foe?Ann Hepatol. 2013 May-Jun;12(3):508-9. Ann Hepatol. 2013. PMID: 23619272 No abstract available.
References
-
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the Prevalence of the Most Common Causes of Chronic Liver Diseases in the United States From 1988 to 2008. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:524.e1–530.e1. - PubMed
-
- Marrero J, Fontana R, Su G, et al. NAFLD May Be a Common Underlying Liver Disease in Patients with Hepatocellular Carcinoma in the United States. Hepatology. 2002;36:1349–1354. - PubMed
-
- Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transplantation. 2012;18:29–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
