Mutations in critical domains confer the human mTOR gene strong tumorigenicity
- PMID: 23322780
- PMCID: PMC3585084
- DOI: 10.1074/jbc.M112.399485
Mutations in critical domains confer the human mTOR gene strong tumorigenicity
Abstract
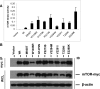
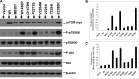
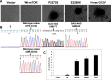
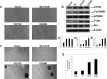
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth, proliferation, and survival. mTOR is frequently activated in human cancers and is a commonly sought anticancer therapeutic target. However, whether the human mTOR gene itself is a proto-oncogene possessing tumorigenicity has not been firmly established. To answer this question, we mutated evolutionarily conserved amino acids, generated eight mutants in the HEAT repeats (M938T) and the FAT (W1456R and G1479N) and kinase (P2273S, V2284M, V2291I, T2294I, and E2288K) domains of mTOR, and studied their oncogenicity. On transient expression in HEK293T cells, these mTOR mutants displayed elevated protein kinase activities accompanied by activated mTOR/p70S6K signaling at varying levels, demonstrating the gain of function of the mTOR gene with these mutations. We selected P2273S and E2288K, the two most catalytically active mutants, to further examine their oncogenicity and tumorigenicity. Stable expression of the two mTOR mutants in NIH3T3 cells strongly activated mTOR/p70S6K signaling, induced cell transformation and invasion, and remarkably, caused rapid tumor formation and growth in athymic nude mice after subcutaneous inoculation of the transfected cells. This study confirms the oncogenic potential of mTOR suggested previously and demonstrates for the first time its tumorigenicity. Thus, beyond the pivotal position of mTOR to relay the oncogenic signals from the upstream phosphatidylinositol 3-kinase/Akt pathway in human cancer, mTOR is capable potentially of playing a direct role in human tumorigenesis if mutated. These results also further support the conclusion that mTOR is a major therapeutic target in human cancers.
Figures



References
-
- Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 - PubMed
-
- Sabatini D. M. (2006) mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer 6, 729–734 - PubMed
-
- Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 - PubMed
-
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

