Transient Influx of nickel in root mitochondria modulates organic acid and reactive oxygen species production in nickel hyperaccumulator Alyssum murale
- PMID: 23322782
- PMCID: PMC3591643
- DOI: 10.1074/jbc.M112.406645
Transient Influx of nickel in root mitochondria modulates organic acid and reactive oxygen species production in nickel hyperaccumulator Alyssum murale
Erratum in
- J Biol Chem. 2013 Aug 9;288(32):23434
Abstract
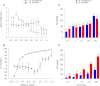
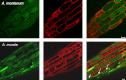
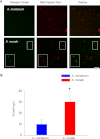
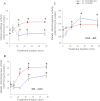
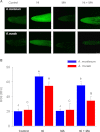
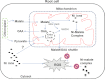
Mitochondria are important targets of metal toxicity and are also vital for maintaining metal homeostasis. Here, we examined the potential role of mitochondria in homeostasis of nickel in the roots of nickel hyperaccumulator plant Alyssum murale. We evaluated the biochemical basis of nickel tolerance by comparing the role of mitochondria in closely related nickel hyperaccumulator A. murale and non-accumulator Alyssum montanum. Evidence is presented for the rapid and transient influx of nickel in root mitochondria of nickel hyperaccumulator A. murale. In an early response to nickel treatment, substantial nickel influx was observed in mitochondria prior to sequestration in vacuoles in the roots of hyperaccumulator A. murale compared with non-accumulator A. montanum. In addition, the mitochondrial Krebs cycle was modulated to increase synthesis of malic acid and citric acid involvement in nickel hyperaccumulation. Furthermore, malic acid, which is reported to form a complex with nickel in hyperaccumulators, was also found to reduce the reactive oxygen species generation induced by nickel. We propose that the interaction of nickel with mitochondria is imperative in the early steps of nickel uptake in nickel hyperaccumulator plants. Initial uptake of nickel in roots results in biochemical responses in the root mitochondria indicating its vital role in homeostasis of nickel ions in hyperaccumulation.
Figures

References
-
- Chaney R. L., Angle J. S., Broadhurst C. L., Peters C. A., Tappero R. V., Sparks D. L. (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J. Environ. Qual. 36, 1429–1443 - PubMed
-
- Baker A. J., Brooks R. R. (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology, and phytochemistry. Biorecovery 1, 81–126
-
- Baker A. J. M., McGrath S. P., Reeves R. D., Smith. J. A. C. (2000) in Phytoremediation of Contaminated Soil and Water (Terry N., Banuelos G., eds) pp. 85–107, Lewis Publishing, Boca Raton, FL
-
- Asemaneh T., Ghaderian S. M., Crawford S. A., Marshall A. T., Baker A. J. (2006) Cellular and subcellular compartmentation of nickel in the Eurasian serpentine plants Alyssum bracteatum, Alyssum murale (Brassicaceae) and Cleome heratensis (Capparaceae). Planta 225, 193–202 - PubMed
-
- Saito A., Saito M., Ichikawa Y., Yoshiba M., Tadano T., Miwa E., Higuchi K. (2010) Difference in the distribution and speciation of cellular nickel between nickel-tolerant and non-tolerant Nicotiana tabacum L. cv. BY-2 cells. Plant Cell Environ. 33, 174–187 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

