Systems-level analysis of proteolytic events in increased vascular permeability and complement activation in skin inflammation
- PMID: 23322905
- PMCID: PMC3872078
- DOI: 10.1126/scisignal.2003512
Systems-level analysis of proteolytic events in increased vascular permeability and complement activation in skin inflammation
Abstract
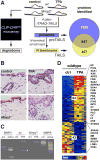
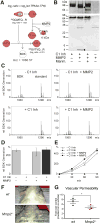
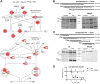
During inflammation, vascular permeability is increased by various proteolytic events, such as the generation of bradykinin, that augment local tissue responses by enabling tissue penetration of serum proteins, including complement and acute-phase proteins. Proteases also govern inflammatory responses by processing extracellular matrix proteins and soluble bioactive mediators. We quantified changes in the proteome and the nature of protein amino termini (the N-terminome) and the altered abundance of murine proteases and inhibitors during skin inflammation. Through analysis of the N-terminome by iTRAQ-TAILS, we identified cotranslational and posttranslational αN-acetylation motifs, quantitative increases in protein abundance, and qualitative changes in the proteolytic signature during inflammation. Of the proteins identified in normal skin, about half were cleaved, and phorbol ester-induced inflammation increased the proportion of cleaved proteins, including chemokines and complement proteins, that were processed at previously uncharacterized sites. In response to phorbol ester-induced inflammation, mice deficient in matrix metalloproteinase 2 (MMP2) showed reduced accumulation of serum proteins in the skin and exhibited different proteolytic networks from those of wild-type mice. We found that the complement 1 (C1) inhibitor attenuated the increase in serum protein accumulation in inflamed skin. Cleavage and inactivation of the C1 inhibitor by MMP2 increased complement activation and bradykinin generation in wild-type mice, leading to increased vessel permeability during inflammation, which was diminished in Mmp2(-/-) mice. Thus, our systems-level analysis of proteolysis dissected cleavage events associated with skin inflammation and demonstrated that loss of a single protease could perturb the proteolytic signaling network and enhance inflammation.
Conflict of interest statement
Figures




Similar articles
-
TAILS N-terminomics of human platelets reveals pervasive metalloproteinase-dependent proteolytic processing in storage.Blood. 2014 Dec 18;124(26):e49-60. doi: 10.1182/blood-2014-04-569640. Epub 2014 Oct 20. Blood. 2014. PMID: 25331112 Free PMC article.
-
Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics.Mol Cell Proteomics. 2010 May;9(5):894-911. doi: 10.1074/mcp.M000050-MCP201. Epub 2010 Mar 20. Mol Cell Proteomics. 2010. PMID: 20305284 Free PMC article.
-
In vivo assessment of protease dynamics in cutaneous wound healing by degradomics analysis of porcine wound exudates.Mol Cell Proteomics. 2015 Feb;14(2):354-70. doi: 10.1074/mcp.M114.043414. Epub 2014 Dec 16. Mol Cell Proteomics. 2015. PMID: 25516628 Free PMC article.
-
The plasma bradykinin-forming pathways and its interrelationships with complement.Mol Immunol. 2010 Aug;47(13):2161-9. doi: 10.1016/j.molimm.2010.05.010. Mol Immunol. 2010. PMID: 20580091 Review.
-
Pathogenesis of Hereditary Angioedema: The Role of the Bradykinin-Forming Cascade.Immunol Allergy Clin North Am. 2017 Aug;37(3):513-525. doi: 10.1016/j.iac.2017.04.001. Immunol Allergy Clin North Am. 2017. PMID: 28687106 Review.
Cited by
-
TAILS N-terminomics and proteomics reveal complex regulation of proteolytic cleavage by O-glycosylation.J Biol Chem. 2018 May 18;293(20):7629-7644. doi: 10.1074/jbc.RA118.001978. Epub 2018 Mar 28. J Biol Chem. 2018. PMID: 29593093 Free PMC article.
-
TAILS N-terminomics of human platelets reveals pervasive metalloproteinase-dependent proteolytic processing in storage.Blood. 2014 Dec 18;124(26):e49-60. doi: 10.1182/blood-2014-04-569640. Epub 2014 Oct 20. Blood. 2014. PMID: 25331112 Free PMC article.
-
Active site specificity profiling datasets of matrix metalloproteinases (MMPs) 1, 2, 3, 7, 8, 9, 12, 13 and 14.Data Brief. 2016 Feb 22;7:299-310. doi: 10.1016/j.dib.2016.02.036. eCollection 2016 Jun. Data Brief. 2016. PMID: 26981551 Free PMC article.
-
Current strategies and findings in clinically relevant post-translational modification-specific proteomics.Expert Rev Proteomics. 2015 Jun;12(3):235-53. doi: 10.1586/14789450.2015.1042867. Epub 2015 May 8. Expert Rev Proteomics. 2015. PMID: 25955281 Free PMC article. Review.
-
Annotating N termini for the human proteome project: N termini and Nα-acetylation status differentiate stable cleaved protein species from degradation remnants in the human erythrocyte proteome.J Proteome Res. 2014 Apr 4;13(4):2028-44. doi: 10.1021/pr401191w. Epub 2014 Mar 10. J Proteome Res. 2014. PMID: 24555563 Free PMC article.
References
-
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. - PubMed
-
- Doucet A, Butler GS, Rodriguez D, Prudova A, Overall CM. Metadegradomics: toward in vivo quantitative degradomics of proteolytic post-translational modifications of the cancer proteome. Mol Cell Proteomics. 2008;7:1925–1951. - PubMed
-
- Lange PF, Overall CM. Protein TAILS: when termini tell tales of proteolysis and function. Curr Opin Chem Biol. 2012 http://dx.doi.org/10.1016/j.cbpa.2012.1011.1025. - DOI - PubMed
-
- Riddel JP, Jr, Aouizerat BE, Miaskowski C, Lillicrap DP. Theories of blood coagulation. J Pediatr Oncol Nurs. 2007;24:123–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

