Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy
- PMID: 23323004
- PMCID: PMC3531690
- DOI: 10.3748/wjg.v18.i47.7026
Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy
Abstract
Aim: To quantitatively assess the ability of double contrast-enhanced ultrasound (DCUS) to detect tumor early response to pre-operative chemotherapy.
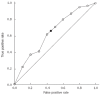
Methods: Forty-three patients with gastric cancer treated with neoadjuvant chemotherapy followed by curative resection between September 2011 and February 2012 were analyzed. Pre-operative chemotherapy regimens of fluorouracil + oxaliplatin or S-1 + oxaliplatin were administered in 2-4 cycles over 6-12 wk periods. All patients underwent contrast-enhanced computed tomography (CT) scan and DCUS before and after two courses of pre-operative chemotherapy. The therapeutic response was assessed by CT using the response evaluation criteria in solid tumors (RECIST 1.1) criteria. Tumor area was assessed by DCUS as enhanced appearance of gastric carcinoma due to tumor vascularity during the contrast phase as compared to the normal gastric wall. Histopathologic analysis was carried out according to the Mandard tumor regression grade criteria and used as the reference standard. Receiver operating characteristic (ROC) analysis was used to evaluate the efficacy of DCUS parameters in differentiating histopathological responders from non-responders.
Results: The study population consisted of 32 men and 11 women, with mean age of 59.7 ± 11.4 years. Neither age, sex, histologic type, tumor site, T stage, nor N stage was associated with pathological response. The responders had significantly smaller mean tumor size than the non-responders (15.7 ± 7.4 cm vs 33.3 ± 14.1 cm, P < 0.01). According to Mandard's criteria, 27 patients were classified as responders, with 11 (40.7%) showing decreased tumor size by DCUS. In contrast, only three (18.8%) of the 16 non-responders showed decreased tumor size by DCUS (P < 0.01). The area under the ROC curve was 0.64, with a 95%CI of 0.46-0.81. The effects of several cut-off points on diagnostic parameters were calculated in the ROC curve analysis. By maximizing Youden's index (sensitivity + specificity - 1), the best cut-off point for distinguishing responders from non-responders was determined, which had optimal sensitivity of 62.9% and specificity of 56.3%. Using this cut-off point, the positive and negative predictive values of DCUS for distinguishing responders from non-responders were 70.8% and 47.4%, respectively. The overall accuracy of DCUS for therapeutic response assessment was 60.5%, slightly higher than the 53.5% for CT response assessment with RECIST criteria (P = 0.663). Although the advantage was not statistically significant, likely due to the small number of cases assessed. DCUS was able to identify decreased perfusion in responders who showed no morphological change by CT imaging, which can be occluded by such treatment effects as fibrosis and edema.
Conclusion: DCUS may represent an innovative tool for more accurately predicting histopathological response to neoadjuvant chemotherapy before surgical resection in patients with locally-advanced gastric cancer.
Keywords: Chemotherapy; Disease management; Gastric cancer; Predictive value of tests; Ultrasonic imaging.
Figures
References
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. - PubMed
-
- Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22:1555–1563. - PubMed
-
- Afaq A, Akin O. Imaging assessment of tumor response: past, present and future. Future Oncol. 2011;7:669–677. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

