The open gate structure of the membrane-embedded KcsA potassium channel viewed from the cytoplasmic side
- PMID: 23323207
- PMCID: PMC3545221
- DOI: 10.1038/srep01063
The open gate structure of the membrane-embedded KcsA potassium channel viewed from the cytoplasmic side
Abstract
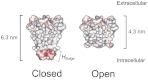
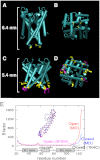
Crystallographic studies of channel proteins have provided insight into the molecular mechanisms of ion channels, even though these structures are obtained in the absence of the membrane and some structural portions have remained unsolved. Here we report the gating structure of the membrane-embedded KcsA potassium channel using atomic force microscopy (AFM). The activation gate of the KcsA channel is located on the intracellular side, and the cytoplasmic domain was truncated to clear the view of this location. Once opened, the individual subunits in the tetramer were resolved with the pore open at the center. Furthermore, AFM was able to capture the previously unsolved bulge helix at the entrance. A molecular dynamics simulation revealed that the bulge helices fluctuated dramatically at the open entryway. This dynamic behavior was observed as vigorous open-channel noise in the single-channel current recordings. The role of the bulge helices in the open gate structure is discussed.
Figures



Similar articles
-
The influence of membrane bilayer thickness on KcsA channel activity.Channels (Austin). 2019 Dec;13(1):424-439. doi: 10.1080/19336950.2019.1676367. Channels (Austin). 2019. PMID: 31608774 Free PMC article.
-
Characterization of the C-terminal domain of a potassium channel from Streptomyces lividans (KcsA).J Biol Chem. 2007 Oct 5;282(40):29163-9. doi: 10.1074/jbc.M703277200. Epub 2007 Aug 10. J Biol Chem. 2007. PMID: 17693406
-
Conformational dynamics of the KcsA potassium channel governs gating properties.Nat Struct Mol Biol. 2007 Nov;14(11):1089-95. doi: 10.1038/nsmb1311. Epub 2007 Oct 7. Nat Struct Mol Biol. 2007. PMID: 17922011 Free PMC article.
-
The potassium channel KcsA and its interaction with the lipid bilayer.Cell Mol Life Sci. 2003 Aug;60(8):1581-90. doi: 10.1007/s00018-003-3172-y. Cell Mol Life Sci. 2003. PMID: 14513833 Free PMC article. Review.
-
Structure of potassium channels.Cell Mol Life Sci. 2015 Oct;72(19):3677-93. doi: 10.1007/s00018-015-1948-5. Epub 2015 Jun 13. Cell Mol Life Sci. 2015. PMID: 26070303 Free PMC article. Review.
Cited by
-
Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2017168118. doi: 10.1073/pnas.2017168118. Proc Natl Acad Sci U S A. 2021. PMID: 33741736 Free PMC article.
-
Cardiolipin binding enhances KcsA channel gating via both its specific and dianion-monoanion interchangeable sites.iScience. 2023 Nov 14;26(12):108471. doi: 10.1016/j.isci.2023.108471. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077151 Free PMC article.
-
The Optimized Conformation Dynamics of the KcsA Filter as a Probe for Lateral Membrane Effects: A First Principle Based Femto-Sec Resolution MD Study.Membranes (Basel). 2022 Nov 24;12(12):1183. doi: 10.3390/membranes12121183. Membranes (Basel). 2022. PMID: 36557090 Free PMC article.
-
Lipid bilayer-atomic force microscopy combined platform records simultaneous electrical and topological changes of the TRP channel polycystin-2 (TRPP2).PLoS One. 2018 Aug 22;13(8):e0202029. doi: 10.1371/journal.pone.0202029. eCollection 2018. PLoS One. 2018. PMID: 30133487 Free PMC article.
-
Regulation of ion channel function by the host lipid bilayer examined by a stopped-flow spectrofluorometric assay.Biophys J. 2014 Mar 4;106(5):1070-8. doi: 10.1016/j.bpj.2014.01.027. Biophys J. 2014. PMID: 24606931 Free PMC article.
References
-
- Hille B. Ion channels of excitable membranes. Sinauer Associates Inc: Sunderland, (2001).
-
- A Doyle D. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998). - PubMed
-
- Jiang Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–22 (2002). - PubMed
-
- Kuo A. et al. Crystal Structure of the potassium channel KirBac1.1 in the Closed State. Science 300, 1922–1926 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

