Novel selective and irreversible mosquito acetylcholinesterase inhibitors for controlling malaria and other mosquito-borne diseases
- PMID: 23323211
- PMCID: PMC3545233
- DOI: 10.1038/srep01068
Novel selective and irreversible mosquito acetylcholinesterase inhibitors for controlling malaria and other mosquito-borne diseases
Abstract
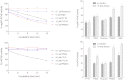
We reported previously that insect acetylcholinesterases (AChEs) could be selectively and irreversibly inhibited by methanethiosulfonates presumably through conjugation to an insect-specific cysteine in these enzymes. However, no direct proof for the conjugation has been published to date, and doubts remain about whether such cysteine-targeting inhibitors have desirable kinetic properties for insecticide use. Here we report mass spectrometric proof of the conjugation and new chemicals that irreversibly inhibited African malaria mosquito AChE with bimolecular inhibition rate constants (k(inact)/K(I)) of 3,604-458,597 M(-1)sec(-1) but spared human AChE. In comparison, the insecticide paraoxon irreversibly inhibited mosquito and human AChEs with k(inact)/K(I) values of 1,915 and 1,507 M(-1)sec(-1), respectively, under the same assay conditions. These results further support our hypothesis that the insect-specific AChE cysteine is a unique and unexplored target to develop new insecticides with reduced insecticide resistance and low toxicity to mammals, fish, and birds for the control of mosquito-borne diseases.
Conflict of interest statement
Y.-P.P., D.D., J.G.P. and S.R. are inventors of a filed patent application that covers the inhibitors disclosed in this article.
Figures

 : reflux.
: reflux.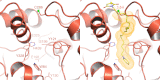

Similar articles
-
Discovery of Species-selective and Resistance-breaking Anticholinesterase Insecticides for the Malaria Mosquito.Curr Med Chem. 2017;24(27):2946-2958. doi: 10.2174/0929867324666170206130024. Curr Med Chem. 2017. PMID: 28176636 Free PMC article. Review.
-
Selective and irreversible inhibitors of mosquito acetylcholinesterases for controlling malaria and other mosquito-borne diseases.PLoS One. 2009 Aug 28;4(8):e6851. doi: 10.1371/journal.pone.0006851. PLoS One. 2009. PMID: 19714254 Free PMC article.
-
Modification of acetylcholinesterase during adaptation to chronic, subacute paraoxon application in rat.Toxicol Appl Pharmacol. 1996 Jan;136(1):20-8. doi: 10.1006/taap.1996.0003. Toxicol Appl Pharmacol. 1996. PMID: 8560475
-
Insect-specific irreversible inhibitors of acetylcholinesterase in pests including the bed bug, the eastern yellowjacket, German and American cockroaches, and the confused flour beetle.Chem Biol Interact. 2010 Sep 6;187(1-3):142-7. doi: 10.1016/j.cbi.2010.01.036. Epub 2010 Jan 28. Chem Biol Interact. 2010. PMID: 20109441
-
Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth.Philos Trans R Soc Lond B Biol Sci. 2013 Jan 6;368(1612):20120429. doi: 10.1098/rstb.2012.0429. Print 2013 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23297352 Free PMC article. Review.
Cited by
-
Acetylcholinesterase target sites for developing environmentally friendly insecticides against Tetranychus urticae (Acari: Tetranychidae).Exp Appl Acarol. 2021 Jun;84(2):419-431. doi: 10.1007/s10493-021-00624-4. Epub 2021 Apr 29. Exp Appl Acarol. 2021. PMID: 33914192
-
Analysis of esterase enzyme activity in adults of the major malaria vector Anopheles funestus.Parasit Vectors. 2016 Feb 27;9:110. doi: 10.1186/s13071-016-1379-7. Parasit Vectors. 2016. PMID: 26920365 Free PMC article.
-
Structure of the G119S Mutant Acetylcholinesterase of the Malaria Vector Anopheles gambiae Reveals Basis of Insecticide Resistance.Structure. 2018 Jan 2;26(1):130-136.e2. doi: 10.1016/j.str.2017.11.021. Epub 2017 Dec 21. Structure. 2018. PMID: 29276037 Free PMC article.
-
Discovery of Species-selective and Resistance-breaking Anticholinesterase Insecticides for the Malaria Mosquito.Curr Med Chem. 2017;24(27):2946-2958. doi: 10.2174/0929867324666170206130024. Curr Med Chem. 2017. PMID: 28176636 Free PMC article. Review.
-
Acetylcholinesterases from the Disease Vectors Aedes aegypti and Anopheles gambiae: Functional Characterization and Comparisons with Vertebrate Orthologues.PLoS One. 2015 Oct 8;10(10):e0138598. doi: 10.1371/journal.pone.0138598. eCollection 2015. PLoS One. 2015. PMID: 26447952 Free PMC article.
References
-
- Miller L. H., Baruch D. I., Marsh K. & Doumbo O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002). - PubMed
-
- Joy D. A. et al. Early origin and recent expansion of Plasmodium falciparum. Science 300, 318–321 (2003). - PubMed
-
- Sachs J. & Malaney P. The economic and social burden of malaria. Nature 415, 680–685 (2002). - PubMed
-
- World Malaria Report 2011. http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf Accessed August 31, 2012.
-
- Fialka J. J. EPA scientists cite pressure in pesticide study. Wall Street Journal, A4. (May25,2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

