An alternative role of FoF1-ATP synthase in Escherichia coli: synthesis of thiamine triphosphate
- PMID: 23323214
- PMCID: PMC3545222
- DOI: 10.1038/srep01071
An alternative role of FoF1-ATP synthase in Escherichia coli: synthesis of thiamine triphosphate
Abstract
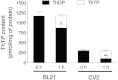
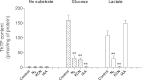
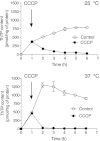
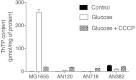
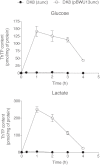
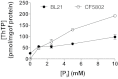
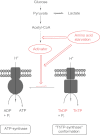
In E. coli, thiamine triphosphate (ThTP), a putative signaling molecule, transiently accumulates in response to amino acid starvation. This accumulation requires the presence of an energy substrate yielding pyruvate. Here we show that in intact bacteria ThTP is synthesized from free thiamine diphosphate (ThDP) and P(i), the reaction being energized by the proton-motive force (Δp) generated by the respiratory chain. ThTP production is suppressed in strains carrying mutations in F(1) or a deletion of the atp operon. Transformation with a plasmid encoding the whole atp operon fully restored ThTP production, highlighting the requirement for F(o)F(1)-ATP synthase in ThTP synthesis. Our results show that, under specific conditions of nutritional downshift, F(o)F(1)-ATP synthase catalyzes the synthesis of ThTP, rather than ATP, through a highly regulated process requiring pyruvate oxidation. Moreover, this chemiosmotic mechanism for ThTP production is conserved from E. coli to mammalian brain mitochondria.
Figures

References
-
- Lakaye B., Wirtzfeld B., Wins P., Grisar T. & Bettendorff L. Thiamine triphosphate, a new signal required for optimal growth of Escherichia coli during amino acid starvation. J. Biol. Chem. 279, 17142–17147 (2004). - PubMed
-
- Bettendorff L. et al. Discovery of a natural thiamine adenine nucleotide. Nat. Chem. Biol. 3, 211–212 (2007). - PubMed
-
- Bettendorff L., Kolb H. A. & Schoffeniels E. Thiamine triphosphate activates an anion channel of large unit conductance in neuroblastoma cells. J. Membr. Biol. 136, 281–288 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

