Predicted heart rate effect of inhaled PF-00610355, a long acting β-adrenoceptor agonist, in volunteers and patients with chronic obstructive pulmonary disease
- PMID: 23323609
- PMCID: PMC3853534
- DOI: 10.1111/bcp.12080
Predicted heart rate effect of inhaled PF-00610355, a long acting β-adrenoceptor agonist, in volunteers and patients with chronic obstructive pulmonary disease
Abstract
Aim: To assess the cardiovascular effects of a new inhaled long-acting β-adrenoceptor agonist PF-00610355 in COPD patients.
Methods: Thirteen thousand and sixty-two heart rate measurements collected in 10 clinical studies from 579 healthy volunteers, asthma and COPD patients were analyzed. The relationship between heart rate profiles and predicted plasma concentration profiles, patient status, demographics and concomitant medication was evaluated using non-linear mixed-effects models. The median heart rate increase in COPD patients for doses of PF-00610355 up to 280 μg once daily was simulated with the final pharmacokinetic/pharmacodynamic (PKPD) model.
Results: An Emax model accounting for delayed on-and off-set of the PF-00610355-induced change in heart rate was developed. The predicted potency in COPD patients was three-fold lower compared with healthy volunteers, while no difference in maximum drug effect was identified. Simulations suggested a maximum placebo-corrected increase of 2.7 (0.90-4.82) beats min(-1) in COPD patients for a PF-00610355 dose of 280 μg once daily, with 19% subjects experiencing a heart rate increase of more than 20 beats min(-1) compared with 8% in the placebo group.
Conclusions: This PKPD analysis supports the clinical observation that no relevant effects of PF-00610355 on heart rate in COPD patients should be expected for doses up to 280 μg once daily.
Keywords: PF-610355; PKPD; long-acting β2 agonist; nonmem; salmeterol; tachycardia.
© 2013 The Authors. British Journal of Clinical Pharmacology © 2013 The British Pharmacological Society.
Figures
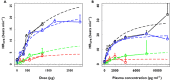
 , human volunteers;
, human volunteers;  , asthma;
, asthma;  , COPD;
, COPD;  , human volunteers, MIAT (Miat monodose inhaler, model 5)
, human volunteers, MIAT (Miat monodose inhaler, model 5)
Similar articles
-
Characterizing systemic exposure of inhaled drugs: application to the long-acting β2-agonist PF-00610355.Clin Pharmacokinet. 2013 Jun;52(6):443-52. doi: 10.1007/s40262-013-0048-7. Clin Pharmacokinet. 2013. PMID: 23494982
-
Vilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD.Pulm Pharmacol Ther. 2013 Apr;26(2):256-64. doi: 10.1016/j.pupt.2012.12.001. Epub 2012 Dec 8. Pulm Pharmacol Ther. 2013. PMID: 23232038
-
Longitudinal FEV1 dose-response model for inhaled PF-00610355 and salmeterol in patients with chronic obstructive pulmonary disease.J Pharmacokinet Pharmacodyn. 2012 Dec;39(6):619-34. doi: 10.1007/s10928-012-9274-0. Epub 2012 Sep 23. J Pharmacokinet Pharmacodyn. 2012. PMID: 23001588
-
Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2019 Mar 6;3(3):CD012930. doi: 10.1002/14651858.CD012930.pub2. Cochrane Database Syst Rev. 2019. PMID: 30839102 Free PMC article.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
Cited by
-
Characterizing systemic exposure of inhaled drugs: application to the long-acting β2-agonist PF-00610355.Clin Pharmacokinet. 2013 Jun;52(6):443-52. doi: 10.1007/s40262-013-0048-7. Clin Pharmacokinet. 2013. PMID: 23494982
-
Toward a Taxonomy for Analyzing the Heart Rate as a Physiological Indicator of Posttraumatic Stress Disorder: Systematic Review and Development of a Framework.JMIR Ment Health. 2020 Jul 22;7(7):e16654. doi: 10.2196/16654. JMIR Ment Health. 2020. PMID: 32706710 Free PMC article. Review.
-
Ultra Long-Acting β-Agonists in Chronic Obstructive Pulmonary Disease.J Exp Pharmacol. 2020 Dec 14;12:589-602. doi: 10.2147/JEP.S259328. eCollection 2020. J Exp Pharmacol. 2020. PMID: 33364854 Free PMC article. Review.
References
-
- Global initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD. 2010. Available at http://www.goldcopd.org/uploads/users/files/GOLDReport_April11%202011.pdf (last accessed 4 January 2012)
-
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1997;10:815–821. - PubMed
-
- Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, Della Cioppa G. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058–1069. - PubMed
-
- Drugs@FDA. FDA Approved Drug Products: Indacaterol. Available at http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/index.cfm (last accessed 10 October 2011)
-
- EMA. European Medicines Agency – Onbrez Breezhaler. 2011. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (last accessed 10 October 2011)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

