The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis
- PMID: 23323860
- PMCID: PMC3715103
- DOI: 10.1111/j.1749-6632.2012.06826.x
The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis
Abstract
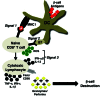
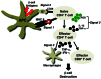
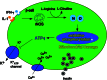
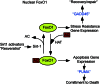
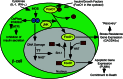
Type 1 diabetes (T1D) is a T cell-mediated autoimmune disease characterized by the destruction of insulin-secreting pancreatic β cells. In humans with T1D and in nonobese diabetic (NOD) mice (a murine model for human T1D), autoreactive T cells cause β-cell destruction, as transfer or deletion of these cells induces or prevents disease, respectively. CD4(+) and CD8(+) T cells use distinct effector mechanisms and act at different stages throughout T1D to fuel pancreatic β-cell destruction and disease pathogenesis. While these adaptive immune cells employ distinct mechanisms for β-cell destruction, one central means for enhancing their autoreactivity is by the secretion of proinflammatory cytokines, such as IFN-γ, TNF-α, and IL-1. In addition to their production by diabetogenic T cells, proinflammatory cytokines are induced by reactive oxygen species (ROS) via redox-dependent signaling pathways. Highly reactive molecules, proinflammatory cytokines are produced upon lymphocyte infiltration into pancreatic islets and induce disease pathogenicity by directly killing β cells, which characteristically possess low levels of antioxidant defense enzymes. In addition to β-cell destruction, proinflammatory cytokines are necessary for efficient adaptive immune maturation, and in the context of T1D they exacerbate autoimmunity by intensifying adaptive immune responses. The first half of this review discusses the mechanisms by which autoreactive T cells induce T1D pathogenesis and the importance of ROS for efficient adaptive immune activation, which, in the context of T1D, exacerbates autoimmunity. The second half provides a comprehensive and detailed analysis of (1) the mechanisms by which cytokines such as IL-1 and IFN-γ influence islet insulin secretion and apoptosis and (2) the key free radicals and transcription factors that control these processes.
© 2013 New York Academy of Sciences.
Figures
References
-
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011;91:79–118. - PubMed
-
- Haskins K, et al. Oxidative stress in type 1 diabetes. Ann. N.Y. Acad. Sci. 2003;1005:43–54. - PubMed
-
- Curtsinger JM, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. - PubMed
-
- Pape KA, et al. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 1997;159:591–598. - PubMed
-
- Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic. Biol. Med. 2004;36:233–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

