Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice
- PMID: 23323935
- PMCID: PMC3647187
- DOI: 10.1111/imm.12071
Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice
Abstract
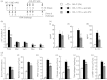
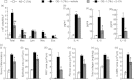
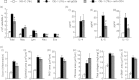
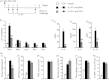
Allergen-specific IgE has long been regarded as a major molecular component of allergic asthma. Additionally, there is increasing evidence of the important roles of interleukin-33 (IL-33) in the disease. Here, we show that IL-33 and alveolar macrophages play essential roles in the exacerbation of IgE-mediated airway inflammation and remodelling. BALB/c mice passively sensitized with ovalbumin (OVA)-specific IgE monoclonal antibody (mAb) were challenged with OVA seven times intratracheally. The seventh challenge exacerbated airway inflammation and remodelling compared with the fourth challenge; furthermore, markedly increased expression of IL-33 in the lungs was observed at the fourth and seventh challenges. When anti-IL-33 or anti-ST2 antibody was administered during the fourth to seventh challenge, airway inflammation and remodelling were significantly inhibited at the seventh challenge. Because increases of IL-33(+) and ST2(+) alveolar macrophages and ST2(+) CD4(+) T cells in the lungs were observed at the fourth challenge, the roles of macrophages and CD4(+) cells were investigated. Depletion of macrophages by 2-chloroadenosine during the fourth to seventh challenge suppressed airway inflammation and remodelling, and IL-33 production in the lung at the seventh challenge; additionally, anti-CD4 mAb inhibited airway inflammation, but not airway remodelling and IL-33 production. Meanwhile, treatment with 2-chloroadenosine or anti-CD4 mAb decreased IL-33-induced airway inflammation in normal mice; airway remodelling was repressed only by 2-chloroadenosine. These results illustrate that macrophage-derived IL-33 contributes to the exacerbation of IgE-mediated airway inflammation by mechanisms associated with macrophages and CD4(+) cells, and airway remodelling through the activation of macrophages.
© 2013 Blackwell Publishing Ltd.
Figures




References
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. - PubMed
-
- Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Cockcroft DW. Allergen-induced increase in nonallergic airway responsiveness: a citation classic revisited. Can Respir J. 2000;7:182–7. - PubMed
-
- Durham SR. The significance of late responses in asthma. Clin Exp Allergy. 1991;21:3–7. - PubMed
-
- Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

