ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling
- PMID: 23324181
- PMCID: PMC4073950
- DOI: 10.1152/ajprenal.00638.2012
ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling
Abstract
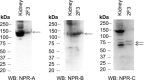
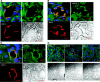
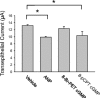
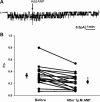
Epithelial sodium channels (ENaCs) located at the apical membrane of polarized epithelial cells are regulated by the second messenger guanosine 3',5'-cyclic monophosphate (cGMP). The mechanism for this regulation has not been completely characterized. Guanylyl cyclases synthesize cGMP in response to various intracellular and extracellular signals. We investigated the regulation of ENaC activity by natriuretic peptide-dependent activation of guanylyl cyclases in Xenopus 2F3 cells. Confocal microscopy studies show natriuretic peptide receptors (NPRs), including those coupled to guanylyl cyclases, are expressed at the apical membrane of 2F3 cells. Single-channel patch-clamp studies using 2F3 cells revealed that atrial natriuretic peptide (ANP) or 8-(4-chlorophenylthio)-cGMP, but not C-type natriuretic peptide or cANP, decreased the open probability of ENaC. This suggests that NPR-A, but not NPR-B or NPR-C, is involved in the natriuretic peptide-mediated regulation of ENaC activity. Also, it is likely that a signaling pathway involving cGMP and nitric oxide (NO) are involved in this mechanism, since inhibitors of soluble guanylyl cyclase, protein kinase G, inducible NO synthase, or an NO scavenger blocked or reduced the effect of ANP on ENaC activity.
Figures




Similar articles
-
Regulation of cGMP by soluble and particulate guanylyl cyclases in cultured human airway smooth muscle.Am J Physiol. 1997 Oct;273(4):L807-13. doi: 10.1152/ajplung.1997.273.4.L807. Am J Physiol. 1997. PMID: 9357856
-
Atrial natriuretic factor and C-type natriuretic peptide induce retraction of human thyrocytes in monolayer culture via guanylyl cyclase receptors.J Endocrinol. 2002 Apr;173(1):169-76. doi: 10.1677/joe.0.1730169. J Endocrinol. 2002. PMID: 11927396
-
Heterogeneous nuclear ribonucleoprotein A1 is a novel cellular target of atrial natriuretic peptide signaling in renal epithelial cells.Cell Signal. 2012 May;24(5):1100-8. doi: 10.1016/j.cellsig.2012.01.006. Epub 2012 Jan 17. Cell Signal. 2012. PMID: 22285803 Free PMC article.
-
Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases.Pharmacol Ther. 2011 Apr;130(1):71-82. doi: 10.1016/j.pharmthera.2010.12.005. Epub 2010 Dec 24. Pharmacol Ther. 2011. PMID: 21185863 Free PMC article. Review.
-
The guanylyl cyclase family of natriuretic peptide receptors.Vitam Horm. 1999;57:123-51. doi: 10.1016/s0083-6729(08)60642-1. Vitam Horm. 1999. PMID: 10232048 Review.
Cited by
-
Sodium retention and volume expansion in nephrotic syndrome: implications for hypertension.Adv Chronic Kidney Dis. 2015 May;22(3):179-84. doi: 10.1053/j.ackd.2014.11.006. Adv Chronic Kidney Dis. 2015. PMID: 25908466 Free PMC article. Review.
-
Pathophysiology and genetics of salt-sensitive hypertension.Front Physiol. 2022 Sep 13;13:1001434. doi: 10.3389/fphys.2022.1001434. eCollection 2022. Front Physiol. 2022. PMID: 36176775 Free PMC article. Review.
-
Atrial Natriuretic Peptide and the Epithelial Sodium Channel Contribute to Spinal Cord Injury-Induced Polyuria in Mice.J Neurotrauma. 2022 May;39(9-10):724-734. doi: 10.1089/neu.2021.0305. J Neurotrauma. 2022. PMID: 35216518 Free PMC article.
-
Effects of elevation of ANP and its deficiency on cardiorenal function.JCI Insight. 2022 May 9;7(9):e148682. doi: 10.1172/jci.insight.148682. JCI Insight. 2022. PMID: 35380994 Free PMC article.
-
Inhibition of Mitochondrial Complex-1 Prevents the Downregulation of NKCC2 and ENaCα in Obstructive Kidney Disease.Sci Rep. 2015 Jul 24;5:12480. doi: 10.1038/srep12480. Sci Rep. 2015. PMID: 26207612 Free PMC article.
References
-
- Alli AA, Gower WR., Jr Characterization of a polyclonal antibody against the cytoplasmic domain of the C-type natriuretic peptide receptor. J Clinical Ligand Assay 31: 3– 7, 2008
-
- Alli AA, Gower WR., Jr The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol 297: C1157– C1167, 2009. - PubMed
-
- Alli AA, Gower WR., Jr Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J Cell Biochem 110: 985– 994, 2010 - PubMed
-
- Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides 26: 1044– 1059, 2005 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

