Chronic electrical stimulation homeostatically decreases spontaneous activity, but paradoxically increases evoked network activity
- PMID: 23324317
- PMCID: PMC3628006
- DOI: 10.1152/jn.00612.2012
Chronic electrical stimulation homeostatically decreases spontaneous activity, but paradoxically increases evoked network activity
Abstract
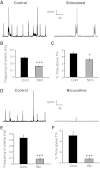
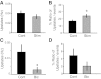
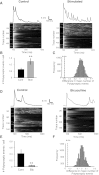
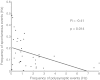
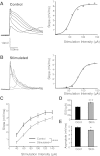
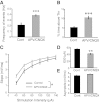
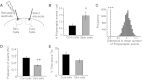
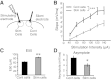
Neural dynamics generated within cortical networks play a fundamental role in brain function. However, the learning rules that allow recurrent networks to generate functional dynamic regimes, and the degree to which these regimes are themselves plastic, are not known. In this study we examined plasticity of network dynamics in cortical organotypic slices in response to chronic changes in activity. Studies have typically manipulated network activity pharmacologically; we used chronic electrical stimulation to increase activity in in vitro cortical circuits in a more physiological manner. Slices were stimulated with "implanted" electrodes for 4 days. Chronic electrical stimulation or treatment with bicuculline decreased spontaneous activity as predicted by homeostatic learning rules. Paradoxically, however, whereas bicuculline decreased evoked network activity, chronic stimulation actually increased the likelihood that evoked stimulation elicited polysynaptic activity, despite a decrease in evoked monosynaptic strength. Furthermore, there was an inverse correlation between spontaneous and evoked activity, suggesting a homeostatic tradeoff between spontaneous and evoked activity. Within-slice experiments revealed that cells close to the stimulated electrode exhibited more evoked polysynaptic activity and less spontaneous activity than cells close to a control electrode. Collectively, our results establish that chronic stimulation changes the dynamic regimes of networks. In vitro studies of homeostatic plasticity typically lack any external input, and thus neurons must rely on "spontaneous" activity to reach homeostatic "set points." However, in the presence of external input we propose that homeostatic learning rules seem to shift networks from spontaneous to evoked regimes.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

