Immunosurveillance against tetraploidization-induced colon tumorigenesis
- PMID: 23324343
- PMCID: PMC3587448
- DOI: 10.4161/cc.23369
Immunosurveillance against tetraploidization-induced colon tumorigenesis
Abstract
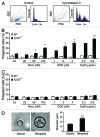
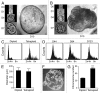
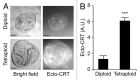
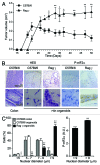
Circumstantial evidence suggests that colon carcinogenesis can ensue the transient tetraploidization of (pre-)malignant cells. In line with this notion, the tumor suppressors APC and TP53, both of which are frequently inactivated in colon cancer, inhibit tetraploidization in vitro and in vivo. Here, we show that-contrarily to their wild-type counterparts- Tp53 (-/-) colonocytes are susceptible to drug-induced or spontaneous tetraploidization in vitro. Colon organoids generated from tetraploid Tp53 (-/-) cells exhibit a close-to-normal morphology as compared to their diploid Tp53 (-/-) counterparts, yet the colonocytes constituting these organoids are characterized by an increased cell size and an elevated expression of the immunostimulatory protein calreticulin on the cell surface. The subcutaneous injection of tetraploid Tp53 (-/-) colon organoids led to the generation of proliferating tumors in immunodeficient, but not immunocompetent, mice. Thus, tetraploid Tp53 (-/-) colonocytes fail to survive in immunocompetent mice and develop neoplastic lesions in immunocompromised settings only. These results suggest that tetraploidy is particularly oncogenic in the context of deficient immunosurveillance.
Keywords: apoptosis; cell cycle; cytochalasin D; mitotic catastrophe; nocodazole; p53.
Figures


Comment in
-
Sensing hyperploidy and immune surveillance: a pas-de-deux.Cell Cycle. 2013 Feb 15;12(4):544-5. doi: 10.4161/cc.23678. Epub 2013 Jan 31. Cell Cycle. 2013. PMID: 23370385 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
