Sorafenib selectively depletes human glioblastoma tumor-initiating cells from primary cultures
- PMID: 23324350
- PMCID: PMC3587450
- DOI: 10.4161/cc.23372
Sorafenib selectively depletes human glioblastoma tumor-initiating cells from primary cultures
Abstract
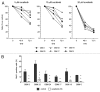
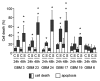
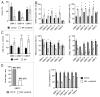
Glioblastomas are grade IV brain tumors characterized by high aggressiveness and invasiveness, giving patients a poor prognosis. We investigated the effects of the multi-kinase inhibitor sorafenib on six cultures isolated from human glioblastomas and maintained in tumor initiating cells-enriching conditions. These cell subpopulations are thought to be responsible for tumor recurrence and radio- and chemo-resistance, representing the perfect target for glioblastoma therapy. Sorafenib reduces proliferation of glioblastoma cultures, and this effect depends, at least in part, on the inhibition of PI3K/Akt and MAPK pathways, both involved in gliomagenesis. Sorafenib significantly induces apoptosis/cell death via downregulation of the survival factor Mcl-1. We provide evidence that sorafenib has a selective action on glioblastoma stem cells, causing enrichment of cultures in differentiated cells, downregulation of the expression of stemness markers required to maintain malignancy (nestin, Olig2 and Sox2) and reducing cell clonogenic ability in vitro and tumorigenic potential in vivo. The selectivity of sorafenib effects on glioblastoma stem cells is confirmed by the lower sensitivity of glioblastoma cultures after differentiation as compared with the undifferentiated counterpart. Since current GBM therapy enriches the tumor in cancer stem cells, the evidence of a selective action of sorafenib on these cells is therapeutically relevant, even if, so far, results from first phase II clinical trials did not demonstrate its efficacy.
Keywords: Mcl-1; glioblastoma; sorafenib; stemness; therapy; tumor initiating cells.
Figures

Similar articles
-
Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3.Mol Cancer Ther. 2010 Apr;9(4):953-62. doi: 10.1158/1535-7163.MCT-09-0947. Epub 2010 Apr 6. Mol Cancer Ther. 2010. PMID: 20371721 Free PMC article.
-
The inhibition of FGF receptor 1 activity mediates sorafenib antiproliferative effects in human malignant pleural mesothelioma tumor-initiating cells.Stem Cell Res Ther. 2017 May 25;8(1):119. doi: 10.1186/s13287-017-0573-7. Stem Cell Res Ther. 2017. PMID: 28545562 Free PMC article.
-
Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT.Eur J Pharmacol. 2018 Aug 5;832:39-49. doi: 10.1016/j.ejphar.2018.05.027. Epub 2018 May 19. Eur J Pharmacol. 2018. PMID: 29782854
-
The Multi-Faceted Effect of Curcumin in Glioblastoma from Rescuing Cell Clearance to Autophagy-Independent Effects.Molecules. 2020 Oct 20;25(20):4839. doi: 10.3390/molecules25204839. Molecules. 2020. PMID: 33092261 Free PMC article. Review.
-
Current clinical development of PI3K pathway inhibitors in glioblastoma.Neuro Oncol. 2012 Jul;14(7):819-29. doi: 10.1093/neuonc/nos117. Epub 2012 May 22. Neuro Oncol. 2012. PMID: 22619466 Free PMC article. Review.
Cited by
-
In vitro and in vivo antiproliferative activity of metformin on stem-like cells isolated from spontaneous canine mammary carcinomas: translational implications for human tumors.BMC Cancer. 2015 Apr 7;15:228. doi: 10.1186/s12885-015-1235-8. BMC Cancer. 2015. PMID: 25884842 Free PMC article.
-
High expression of leptin receptor leads to temozolomide resistance with exhibiting stem/progenitor cell features in gliobalastoma.Cell Cycle. 2013 Dec 15;12(24):3833-40. doi: 10.4161/cc.26809. Epub 2013 Oct 16. Cell Cycle. 2013. PMID: 24131927 Free PMC article.
-
The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma.Cell Cycle. 2015;14(21):3418-29. doi: 10.1080/15384101.2015.1090063. Cell Cycle. 2015. PMID: 26566863 Free PMC article.
-
Inhibition of Chloride Intracellular Channel 1 (CLIC1) as Biguanide Class-Effect to Impair Human Glioblastoma Stem Cell Viability.Front Pharmacol. 2018 Aug 21;9:899. doi: 10.3389/fphar.2018.00899. eCollection 2018. Front Pharmacol. 2018. PMID: 30186163 Free PMC article.
-
Repurposed Biguanide Drugs in Glioblastoma Exert Antiproliferative Effects via the Inhibition of Intracellular Chloride Channel 1 Activity.Front Oncol. 2019 Mar 13;9:135. doi: 10.3389/fonc.2019.00135. eCollection 2019. Front Oncol. 2019. PMID: 30918838 Free PMC article. Review.
References
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
