Developmentally regulated expression and complex processing of barley pri-microRNAs
- PMID: 23324356
- PMCID: PMC3558349
- DOI: 10.1186/1471-2164-14-34
Developmentally regulated expression and complex processing of barley pri-microRNAs
Abstract
Background: MicroRNAs (miRNAs) regulate gene expression via mRNA cleavage or translation inhibition. In spite of barley being a cereal of great economic importance, very little data is available concerning its miRNA biogenesis. There are 69 barley miRNA and 67 pre-miRNA sequences available in the miRBase (release 19). However, no barley pri-miRNA and MIR gene structures have been shown experimentally. In the present paper, we examine the biogenesis of selected barley miRNAs and the developmental regulation of their pri-miRNA processing to learn more about miRNA maturation in barely.
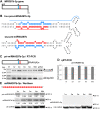
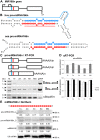
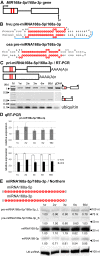
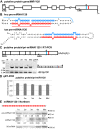
Results: To investigate the organization of barley microRNA genes, nine microRNAs - 156g, 159b, 166n, 168a-5p/168a-3p, 171e, 397b-3p, 1120, and 1126 - were selected. Two of the studied miRNAs originate from one MIR168a-5p/168a-3p gene. The presence of all miRNAs was confirmed using a Northern blot approach. The miRNAs are encoded by genes with diverse organizations, representing mostly independent transcription units with or without introns. The intron-containing miRNA transcripts undergo complex splicing events to generate various spliced isoforms. We identified miRNAs that were encoded within introns of the noncoding genes MIR156g and MIR1126. Interestingly, the intron that encodes miR156g is spliced less efficiently than the intron encoding miR1126 from their specific precursors. miR397b-3p was detected in barley as a most probable functional miRNA, in contrast to rice where it has been identified as a complementary partner miRNA*. In the case of miR168a-5p/168a-3p, we found the generation of stable, mature molecules from both pre-miRNA arms, confirming evolutionary conservation of the stability of both species, as shown in rice and maize. We suggest that miR1120, located within the 3' UTR of a protein-coding gene and described as a functional miRNA in wheat, may represent a siRNA generated from a mariner-like transposable element.
Conclusions: Seven of the eight barley miRNA genes characterized in this study contain introns with their respective transcripts undergoing developmentally specific processing events prior to the dicing out of pre-miRNA species from their pri-miRNA precursors. The observed tendency to maintain the intron encoding miR156g within the transcript, and preferences in splicing the miR1126-harboring intron, may suggest the existence of specific regulation of the levels of intron-derived miRNAs in barley.
Figures




Similar articles
-
Transcriptionally and post-transcriptionally regulated microRNAs in heat stress response in barley.J Exp Bot. 2014 Nov;65(20):6123-35. doi: 10.1093/jxb/eru353. Epub 2014 Sep 2. J Exp Bot. 2014. PMID: 25183744 Free PMC article.
-
New insights into pri-miRNA processing and accumulation in plants.Wiley Interdiscip Rev RNA. 2015 Sep-Oct;6(5):533-45. doi: 10.1002/wrna.1292. Epub 2015 Jun 29. Wiley Interdiscip Rev RNA. 2015. PMID: 26119101 Review.
-
A computational-based update on microRNAs and their targets in barley (Hordeum vulgare L.).BMC Genomics. 2010 Oct 22;11:595. doi: 10.1186/1471-2164-11-595. BMC Genomics. 2010. PMID: 20969764 Free PMC article.
-
Gene structures and processing of Arabidopsis thaliana HYL1-dependent pri-miRNAs.Nucleic Acids Res. 2009 May;37(9):3083-93. doi: 10.1093/nar/gkp189. Epub 2009 Mar 20. Nucleic Acids Res. 2009. PMID: 19304749 Free PMC article.
-
Cross talk between spliceosome and microprocessor defines the fate of pre-mRNA.Wiley Interdiscip Rev RNA. 2014 Sep-Oct;5(5):647-58. doi: 10.1002/wrna.1236. Epub 2014 Apr 30. Wiley Interdiscip Rev RNA. 2014. PMID: 24788135 Review.
Cited by
-
The liverwort Pellia endiviifolia shares microtranscriptomic traits that are common to green algae and land plants.New Phytol. 2015 Apr;206(1):352-367. doi: 10.1111/nph.13220. Epub 2014 Dec 19. New Phytol. 2015. PMID: 25530158 Free PMC article.
-
The plastid-nucleus located DNA/RNA binding protein WHIRLY1 regulates microRNA-levels during stress in barley (Hordeum vulgare L.).RNA Biol. 2018;15(7):886-891. doi: 10.1080/15476286.2018.1481695. Epub 2018 Jul 14. RNA Biol. 2018. PMID: 29947287 Free PMC article.
-
What Do We Know about Barley miRNAs?Int J Mol Sci. 2022 Nov 25;23(23):14755. doi: 10.3390/ijms232314755. Int J Mol Sci. 2022. PMID: 36499082 Free PMC article.
-
Wheat miRNA ancestors: evident by transcriptome analysis of A, B, and D genome donors.Funct Integr Genomics. 2017 May;17(2-3):171-187. doi: 10.1007/s10142-016-0487-y. Epub 2016 Mar 31. Funct Integr Genomics. 2017. PMID: 27032785
-
MiRNAs differentially expressed in vegetative and reproductive organs of Marchantia polymorpha - insights into their expression pattern, gene structures and function.RNA Biol. 2024 Jan;21(1):1-12. doi: 10.1080/15476286.2024.2303555. Epub 2024 Feb 1. RNA Biol. 2024. PMID: 38303117 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

