Differences in degradation lead to asynchronous expression of cyclin E1 and cyclin E2 in cancer cells
- PMID: 23324394
- PMCID: PMC3594260
- DOI: 10.4161/cc.23409
Differences in degradation lead to asynchronous expression of cyclin E1 and cyclin E2 in cancer cells
Abstract
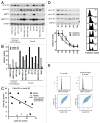
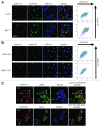
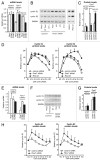
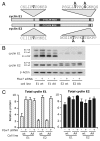
Cyclin E1 is expressed at the G 1/S phase transition of the cell cycle to drive the initiation of DNA replication and is degraded during S/G2M. Deregulation of its periodic degradation is observed in cancer and is associated with increased proliferation and genomic instability. We identify that in cancer cells, unlike normal cells, the closely related protein cyclin E2 is expressed predominantly in S phase, concurrent with DNA replication. This occurs at least in part because the ubiquitin ligase component that is responsible for cyclin E1 downregulation in S phase, Fbw7, fails to effectively target cyclin E2 for proteosomal degradation. The distinct cell cycle expression of the two E-type cyclins in cancer cells has implications for their roles in genomic instability and proliferation and may explain their associations with different signatures of disease.
Keywords: Fbw7; cell cycle; cyclin E1; cyclin E2; proliferation.
Figures


References
-
- Juan G, Cordon-Cardo C. Intranuclear compartmentalization of cyclin E during the cell cycle: disruption of the nucleoplasm-nucleolar shuttling of cyclin E in bladder cancer. Cancer Res. 2001;61:1220–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
