Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations
- PMID: 23324461
- PMCID: PMC3585029
- DOI: 10.4161/nucl.23388
Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations
Abstract
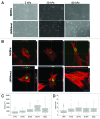
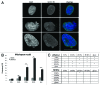
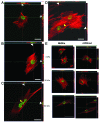
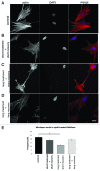
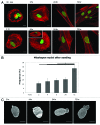
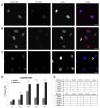
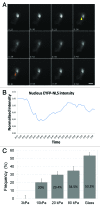
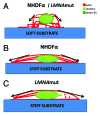
Laminopathies, mainly caused by mutations in the LMNA gene, are a group of inherited diseases with a highly variable penetrance; i.e., the disease spectrum in persons with identical LMNA mutations range from symptom-free conditions to severe cardiomyopathy and progeria, leading to early death. LMNA mutations cause nuclear abnormalities and cellular fragility in response to cellular mechanical stress, but the genotype/phenotype correlations in these diseases remain unclear. Consequently, tools such as mutation analysis are not adequate for predicting the course of the disease. Here, we employ growth substrate stiffness to probe nuclear fragility in cultured dermal fibroblasts from a laminopathy patient with compound progeroid syndrome. We show that culturing of these cells on substrates with stiffness higher than 10 kPa results in malformations and even rupture of the nuclei, while culture on a soft substrate (3 kPa) protects the nuclei from morphological alterations and ruptures. No malformations were seen in healthy control cells at any substrate stiffness. In addition, analysis of the actin cytoskeleton organization in this laminopathy cells demonstrates that the onset of nuclear abnormalities correlates to an increase in cytoskeletal tension. Together, these data indicate that culturing of these LMNA mutated cells on substrates with a range of different stiffnesses can be used to probe the degree of nuclear fragility. This assay may be useful in predicting patient-specific phenotypic development and in investigations on the underlying mechanisms of nuclear and cellular fragility in laminopathies.
Keywords: lamina; laminopathies; nuclear rupture; nuclear shape alteration; substrate stiffness.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
