A mixture of functionally oligoclonal humanized monoclonal antibodies that neutralize Clostridium difficile TcdA and TcdB with high levels of in vitro potency shows in vivo protection in a hamster infection model
- PMID: 23324518
- PMCID: PMC3592348
- DOI: 10.1128/CVI.00625-12
A mixture of functionally oligoclonal humanized monoclonal antibodies that neutralize Clostridium difficile TcdA and TcdB with high levels of in vitro potency shows in vivo protection in a hamster infection model
Abstract
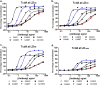
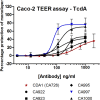
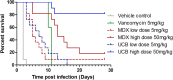
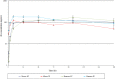
Clostridium difficile infections are a major cause of antibiotic-associated diarrhea in hospital and care facility patients. In spite of the availability of effective antibiotic treatments, C. difficile infection (CDI) is still a major cause of patient suffering, death, and substantial health care costs. Clostridium difficile exerts its major pathological effects through the actions of two protein exotoxins, TcdA and TcdB, which bind to and disrupt gut tissue. Antibiotics target the infecting bacteria but not the exotoxins. Administering neutralizing antibodies against TcdA and TcdB to patients receiving antibiotic treatment might modulate the effects of the exotoxins directly. We have developed a mixture of three humanized IgG1 monoclonal antibodies (MAbs) which neutralize TcdA and TcdB to address three clinical needs: reduction of the severity and duration of diarrhea, reduction of death rates, and reduction of the rate of recurrence. The UCB MAb mixture showed higher potency in a variety of in vitro binding and neutralization assays (∼10-fold improvements), higher levels of protection in a hamster model of CDI (82% versus 18% at 28 days), and higher valencies of toxin binding (12 versus 2 for TcdA and 3 versus 2 for TcdB) than other agents in clinical development. Comparisons of the MAb properties also offered some insight into the potential relative importance of TcdA and TcdB in the disease process.
Figures

References
-
- Bauer MP, Notermans DW, Van Benthem BHB, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, Van Dissel JT, Kuijper EJ. 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73 - PubMed
-
- Wullt M, Odenholt I. 2004. A double-blind randomized controlled trial of fusidic acid and metronidazole for treatment of an initial episode of Clostridium difficile-associated diarrhoea. J. Antimicrob. Chemother. 54:211–216 - PubMed
-
- Oake N, Taljaard M, Van Walraven C, Wilson K, Roth V, Forster AJ. 2010. The effect of hospital-acquired Clostridium difficile infection on in-hospital mortality. Arch. Intern. Med. 170:1804–1810 - PubMed
-
- O'Brien JA, Lahue BJ, Caro JJ, Davidson DM. 2007. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect. Control Hosp. Epidemiol. 28:1219–1227 - PubMed
-
- Dubberke ER, Wertheimer AI. 2009. Review of current literature on the economic burden of Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 30:57–66 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

