Fel d 1-airway inflammation prevention and treatment by co-immunization vaccine via induction of CD4+CD25-Foxp3+ Treg cells
- PMID: 23324570
- PMCID: PMC3899136
- DOI: 10.4161/hv.23518
Fel d 1-airway inflammation prevention and treatment by co-immunization vaccine via induction of CD4+CD25-Foxp3+ Treg cells
Abstract
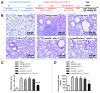
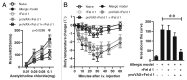
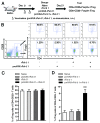
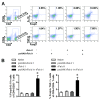
Pet allergens are major causes for asthma and allergic rhinitis. Fel d 1 protein, a key pet allergen from domestic cat, can sensitize host and trigger asthma attack. In this study, we report that co-immunization with recombinant Fel d 1 protein (rFel d 1) plus plasmid DNA that contains Fe1 d 1 gene was effective in preventing and treating the natural Fel d 1 (nFel d 1) induced allergic airway inflammation in mice. A population of T regulatory cells (iTreg) exhibiting a CD4+CD25-Foxp3+ phenotype and expressing IL-10 and TGF-β was induced by this co-immunization strategy. Furthermore, after adoptive transfers of the iTreg cells, mice that were pre-sensitized and challenged with nFel d 1 exhibited less signs of allergic inflammation, AHR and a reduced allergic immune response. These data indicate that co-immunization with DNA and protein mixture vaccine may be an effective treatment for cat allergy.
Keywords: Fel d 1; T regulatory cell; airway inflammation; co-immunization; vaccine.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
