The archaeal DnaG protein needs Csl4 for binding to the exosome and enhances its interaction with adenine-rich RNAs
- PMID: 23324612
- PMCID: PMC3672285
- DOI: 10.4161/rna.23450
The archaeal DnaG protein needs Csl4 for binding to the exosome and enhances its interaction with adenine-rich RNAs
Abstract
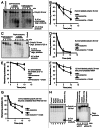
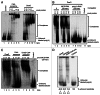
The archaeal RNA-degrading exosome contains a catalytically active hexameric core, an RNA-binding cap formed by Rrp4 and Csl4 and the protein annotated as DnaG (bacterial type primase) with so-far-unknown functions in RNA metabolism. We found that the archaeal DnaG binds to the Csl4-exosome but not to the Rrp4-exosome of Sulfolobus solfataricus. In vitro assays revealed that DnaG is a poly(A)-binding protein enhancing the degradation of adenine-rich transcripts by the Csl4-exosome. DnaG is the second poly(A)-binding protein besides Rrp4 in the heteromeric, RNA-binding cap of the S. solfataricus exosome. This apparently reflects the need for effective and selective recruitment of adenine-rich RNAs to the exosome in the RNA metabolism of S. solfataricus.
Keywords: DnaG; RNA binding; archaea; exosome; poly(A) binding.
Figures



References
-
- Farhoud MH, Wessels HJ, Steenbakkers PJ, Mattijssen S, Wevers RA, van Engelen BG, et al. Protein complexes in the archaeon Methanothermobacter thermautotrophicus analyzed by blue native/SDS-PAGE and mass spectrometry. Mol Cell Proteomics. 2005;4:1653–63. doi: 10.1074/mcp.M500171-MCP200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
