Sequence and immunogenicity of a clinically approved novel measles virus vaccine vector
- PMID: 23324616
- PMCID: PMC3891718
- DOI: 10.4161/hv.23242
Sequence and immunogenicity of a clinically approved novel measles virus vaccine vector
Abstract
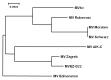
The measles virus vaccine (MVbv) is a clinically certified and well-tolerated vaccine strain that has been given both parenterally and mucosally. It has been extensively used in children and has proven to be safe and effective in eliciting protective immunity. This specific strain was therefore chosen to generate a measles viral vector. The genome of the commercial MVbv vaccine strain was isolated, sequenced and a plasmid, p(+)MVb, enabling transcription of the viral antigenome and rescue of MVb, was constructed. Phylogenic and phenotypic analysis revealed that MVbv and the rescued MVb constitute another evolutionary branch within the hitherto classified measles vaccines. Plasmid p(+)MVb was modified by insertion of artificial MV-type transcription units (ATUs) for the generation of recombinant viruses (rMVb) expressing additional proteins. Replication characteristics and immunogenicity of rMVb vectors were similar to the parental MVbv and to other vaccine strains. The expression of the additional proteins was stable over 10 serial virus transfers, which corresponds to an amplification greater than 10 ( 20) . The excellent safety record and its efficient application as aerosol may add to the usefulness of the derived vectors.
Keywords: live-attenuated vaccines; recombinant measles virus; viral vectors.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
